Effect of simulated acid rain on the stability of calcium carbonate immobilized by microbial carbonate precipitation - ScienceDirect

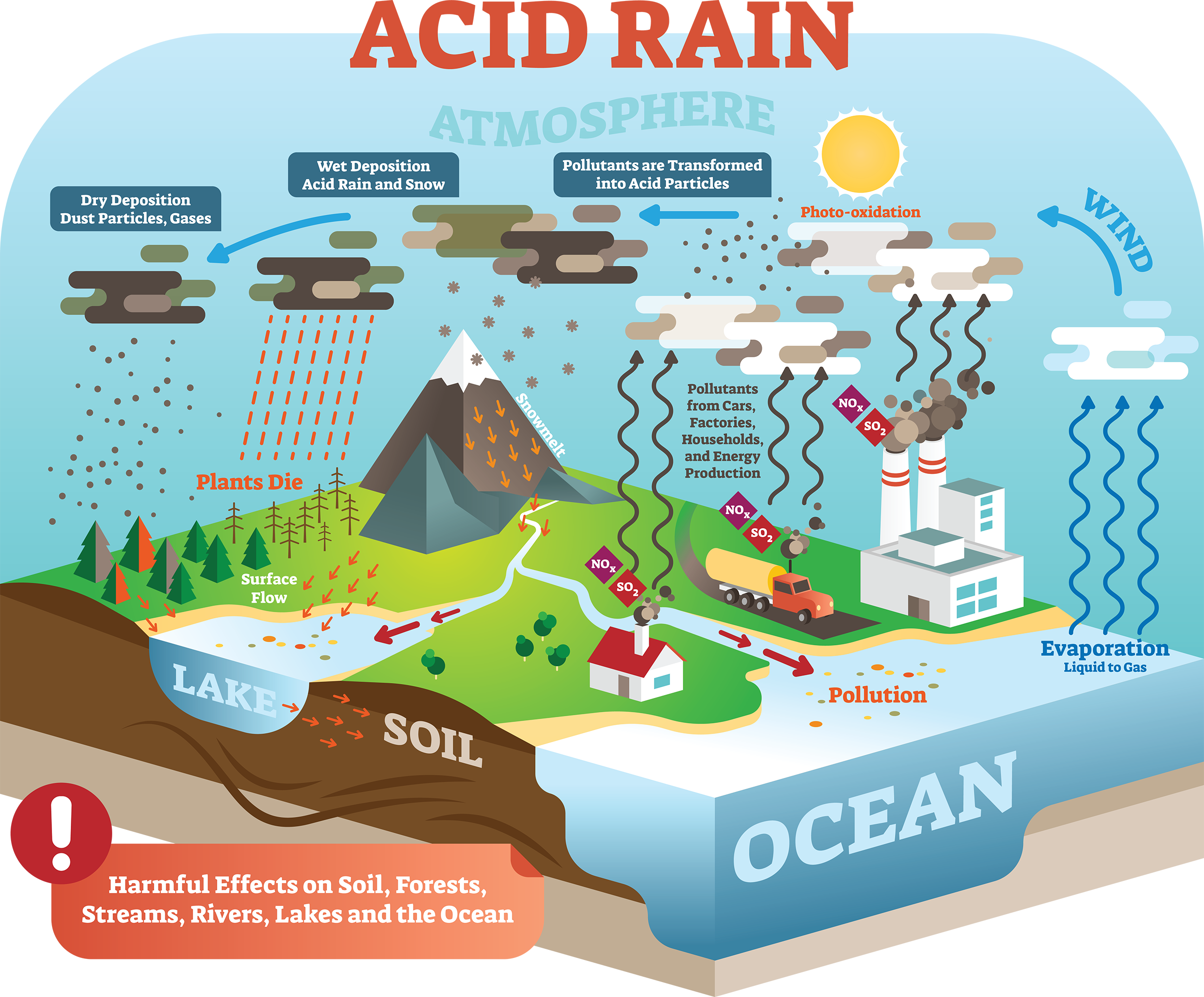
Acids, Bases and Salts Acids give up hydrogen ions (H+) in a water solution. Bases give up hydroxide ions (OH-) in a water solution. Mullis. - ppt video online download
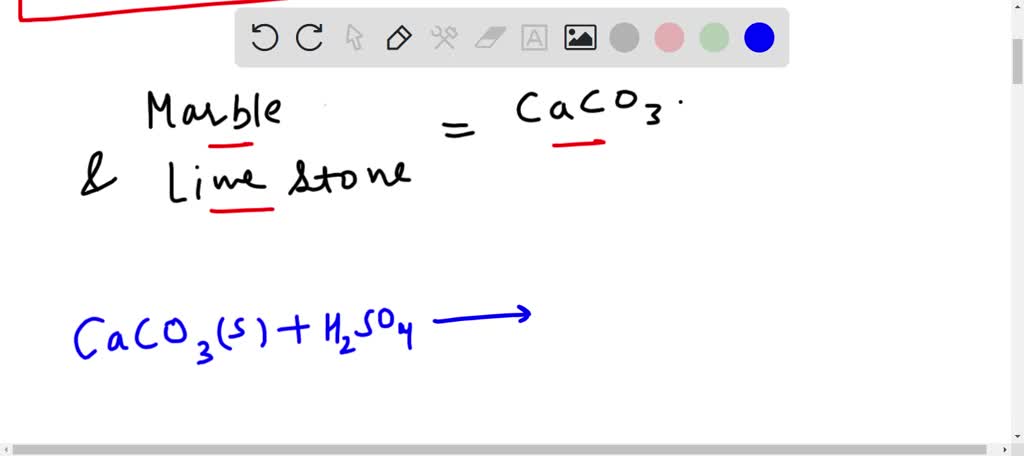
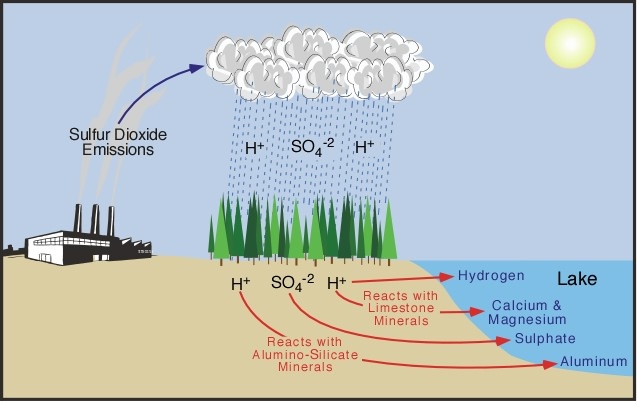
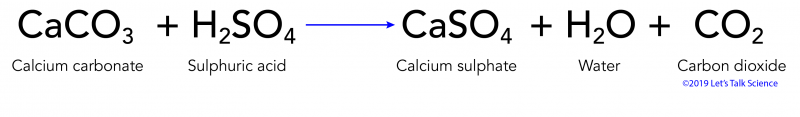
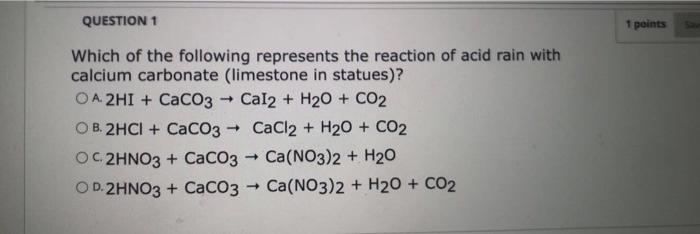
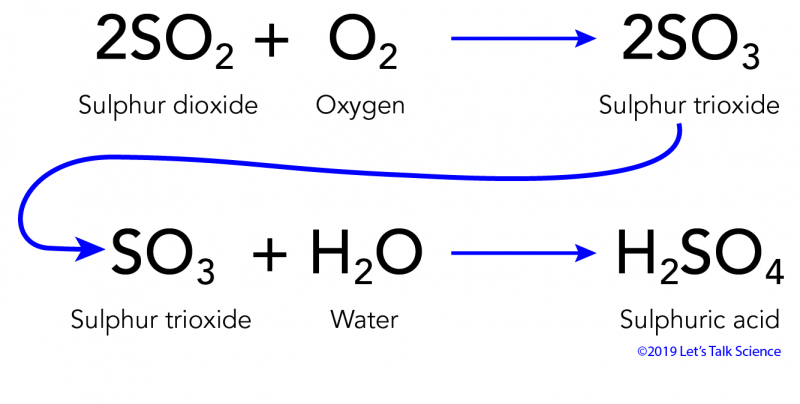
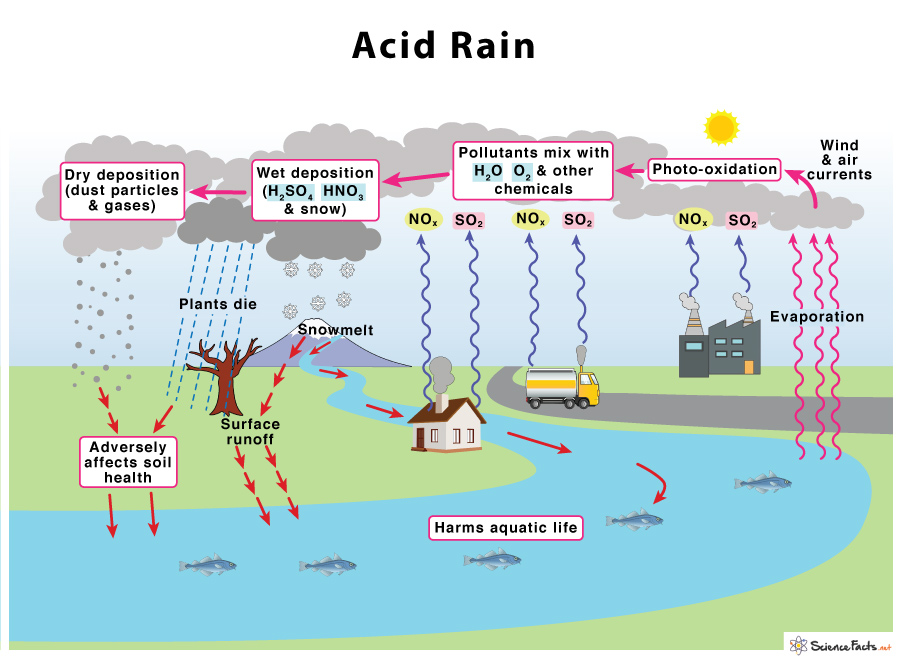
SOLVED: Write an equation to show how sulfuric acids in acid rain reacts with marble and limestone. (Both marble and limestone are primarily calcium carbonate.)
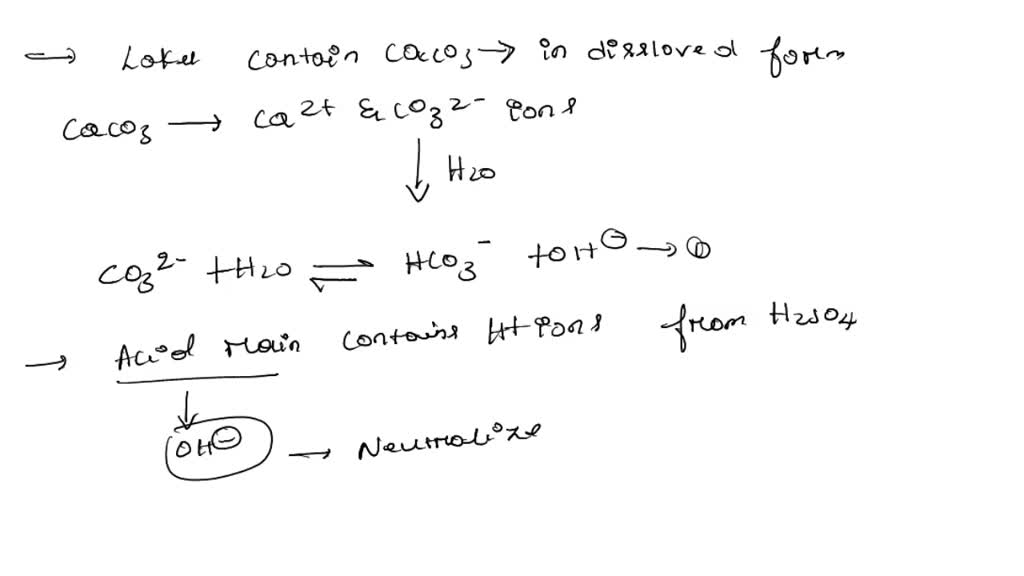
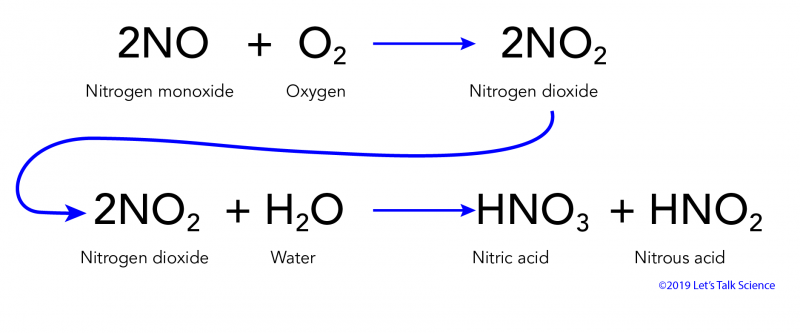
SOLVED: Lakes have a natural buffering capacity, especially in regions where limestone gives rise to dissolved calcium carbonate. Write an equation for the effect of a small amount of acid rain containing