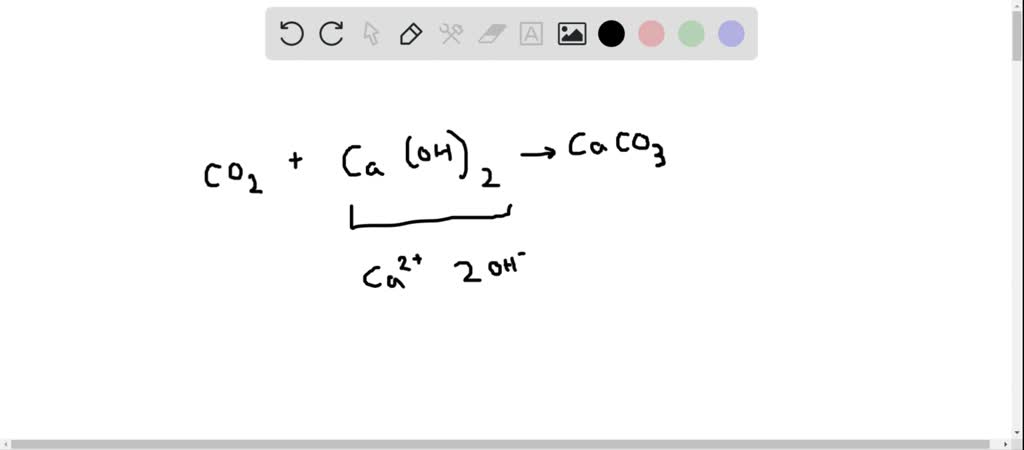
Ca(OH)2+CO2 =CaCO3 +H2O Balanced Equation|Calcium hydroxide+Carbon dioxide= Calcium carbonate + Water - YouTube

Reactions of carbon dioxide - Gas chemistry - (CCEA) - GCSE Chemistry (Single Science) Revision - CCEA - BBC Bitesize

CO2 capture and ions removal through reaction with potassium hydroxide in desalination reject brine: Statistical optimization - ScienceDirect
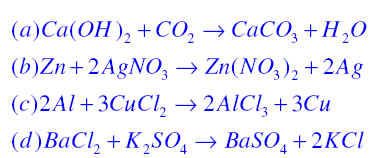
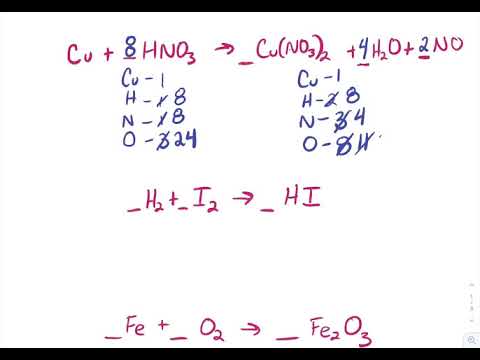
Write the balanced chemical equations for the following reactions. (a) Calcium hydroxide + Carbon dioxide → Calcium carbonate + Water | Pushpender86's Blog

Optimization of the structural characteristics of CaO and its effective stabilization yield high-capacity CO2 sorbents | Nature Communications

Write the balanced chemical equations for the following reactions.A Calcium hydroxide + Carbon dioxide → Calcium carbonate + waterB Zinc + Silver nitrate → Zinc nitrate + SilverC Aluminium + copper chloride

SOLVED:Write balanced chemical equations for each of the following processes: (a) Calcium phosphate reacts with sulfuric acid to produce calcium sulfate and phosphoric acid. (b) Calcium phosphate reacts with water containing dissolved
SOLVED:When carbon dioxide is bubbled through a clear calcium hydroxide solution, the solution appears milky. Write an equation for the reaction, and explain how this reaction illustrates that CO2 is an acidic
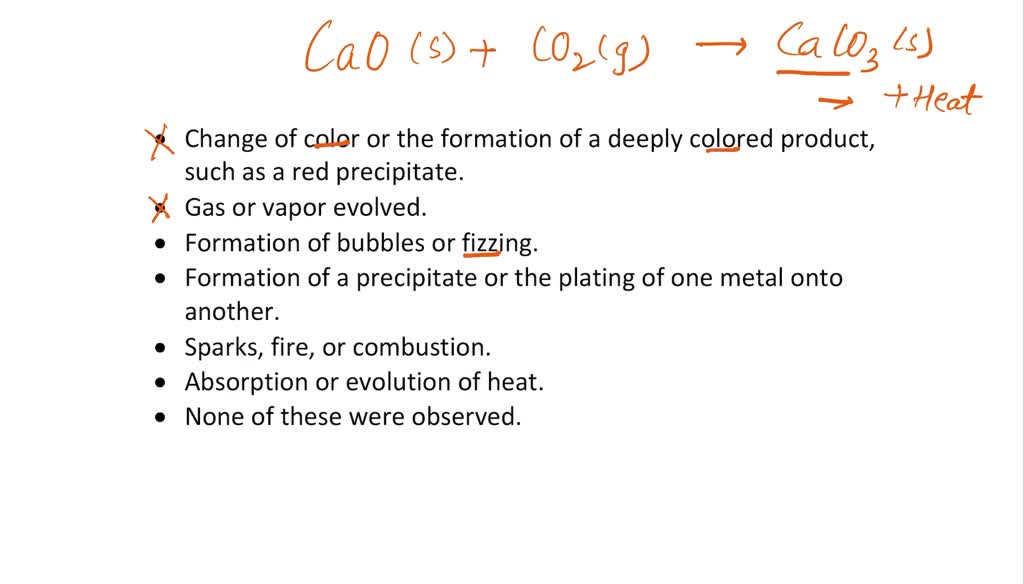
SOLVED: Reaction of calcium oxide and carbon dioxide: Watch the video for this experiment and list each of the changes you observed. Change of color or the formation of deeply colored product

SciELO - Brazil - Comparative evaluation of the pH of calcium hydroxide powder in contact with carbon dioxide (CO2) Comparative evaluation of the pH of calcium hydroxide powder in contact with carbon



![SQP] A clear solution of slaked lime is made by dissolving Ca(OH)2 SQP] A clear solution of slaked lime is made by dissolving Ca(OH)2](https://d1avenlh0i1xmr.cloudfront.net/edaba2bb-20df-4980-9a2a-d6fc77a0b818/reaction-of-slaked-lime-with-co2---teachoo.jpg)