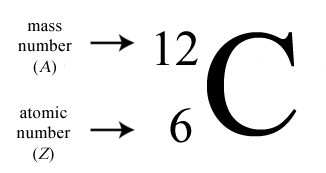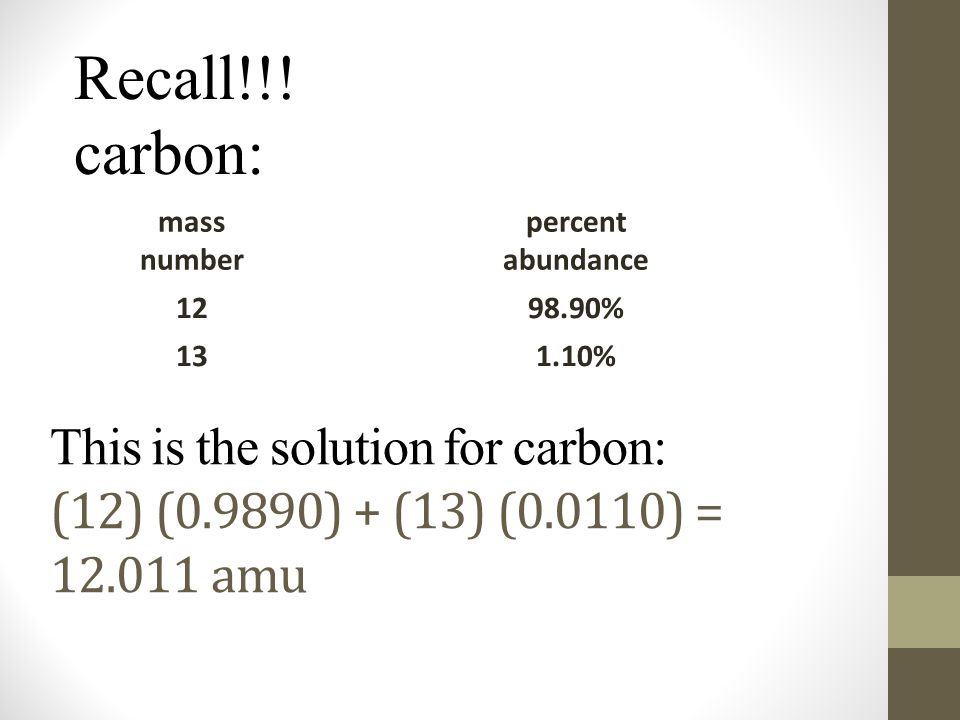
This is the solution for carbon: (12) (0.9890) + (13) (0.0110) = amu mass number percent abundance % % Recall!!! carbon: - ppt download

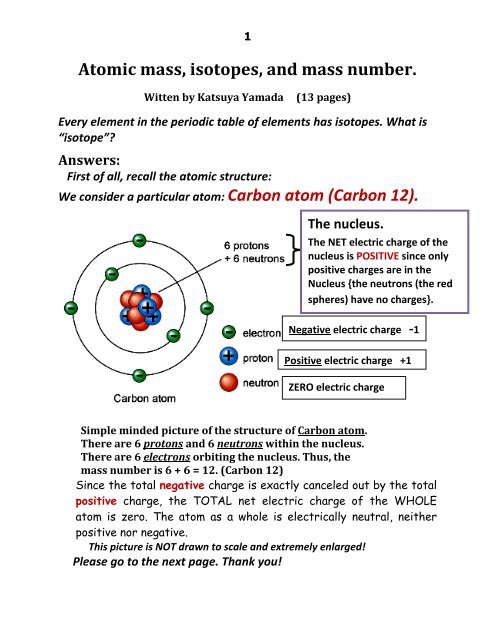
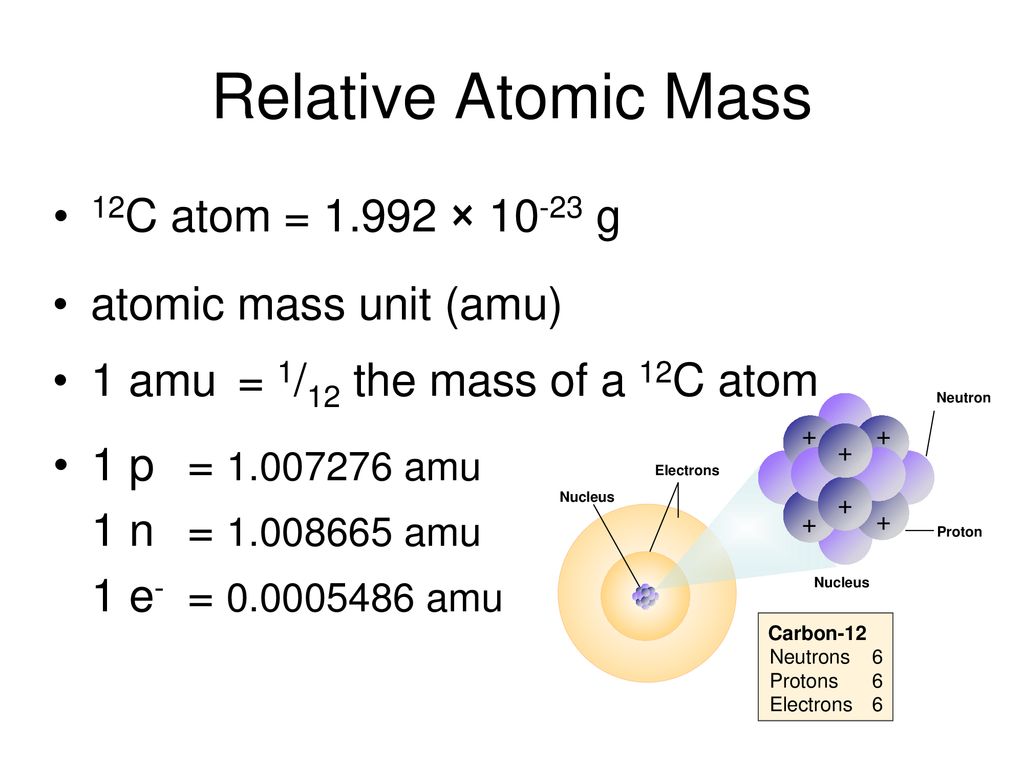
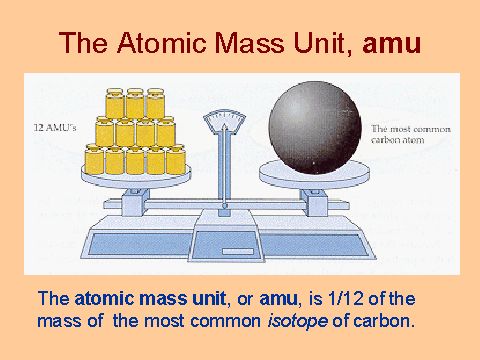
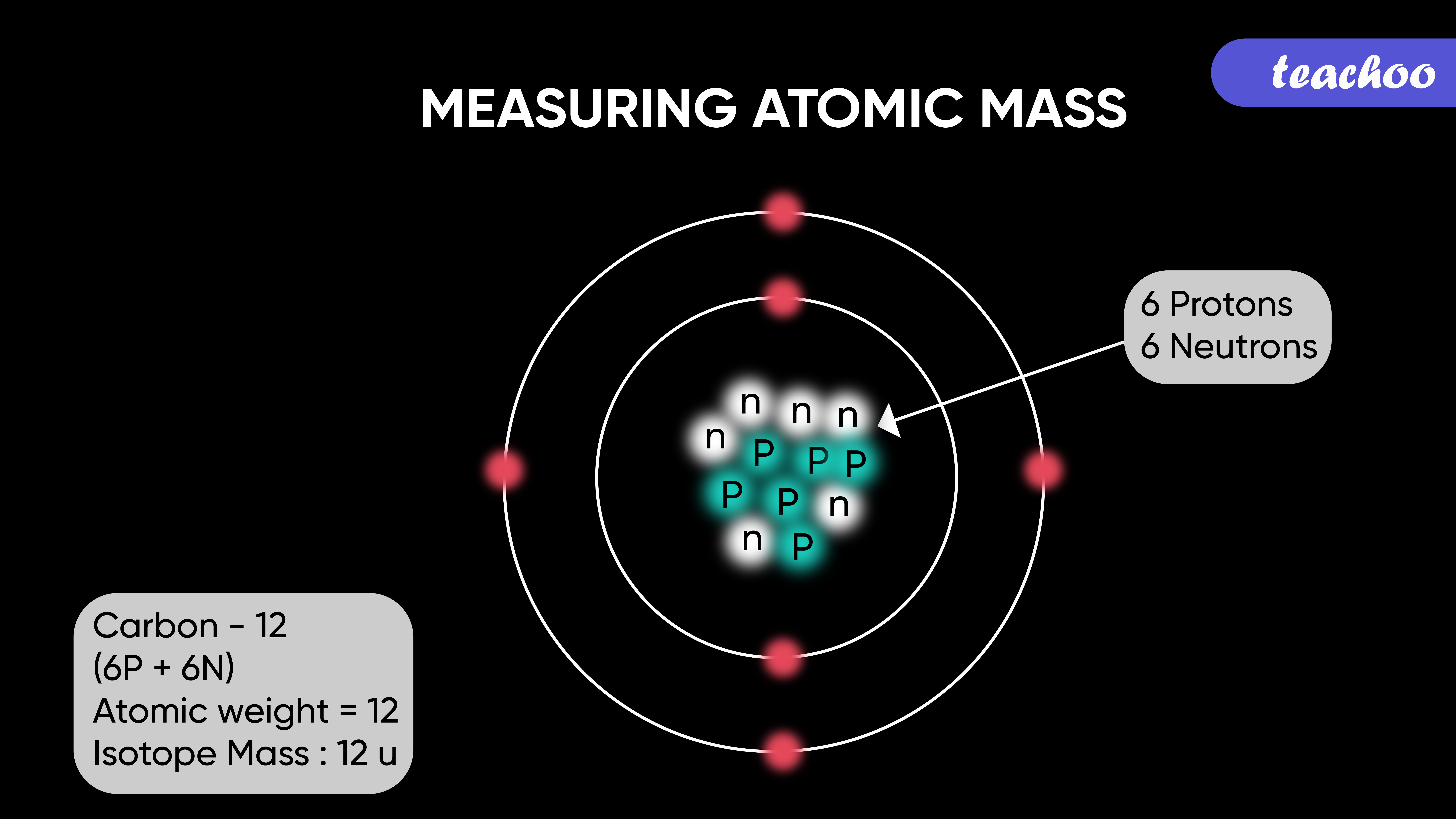
Atomic Mass Standard mass unit is derived from carbon 12 Atomic mass unit – the mass equal to 1/12 the mass of one Carbon 12 atom. - ppt download
ntif we consider that 1/6, in place of 1/12,mass of carbon atom is taken to be relative atomic mass unit ,the mass of 1 mole of substance will?n

Atomic Mass Standard mass unit is derived from carbon 12 Atomic mass unit – the mass equal to 1/12 the mass of one Carbon 12 atom. - ppt download

Carbon occur in nature as a mixture of `C-12` and `C-13`. Average atomic mass of carbon is `12.011 - YouTube