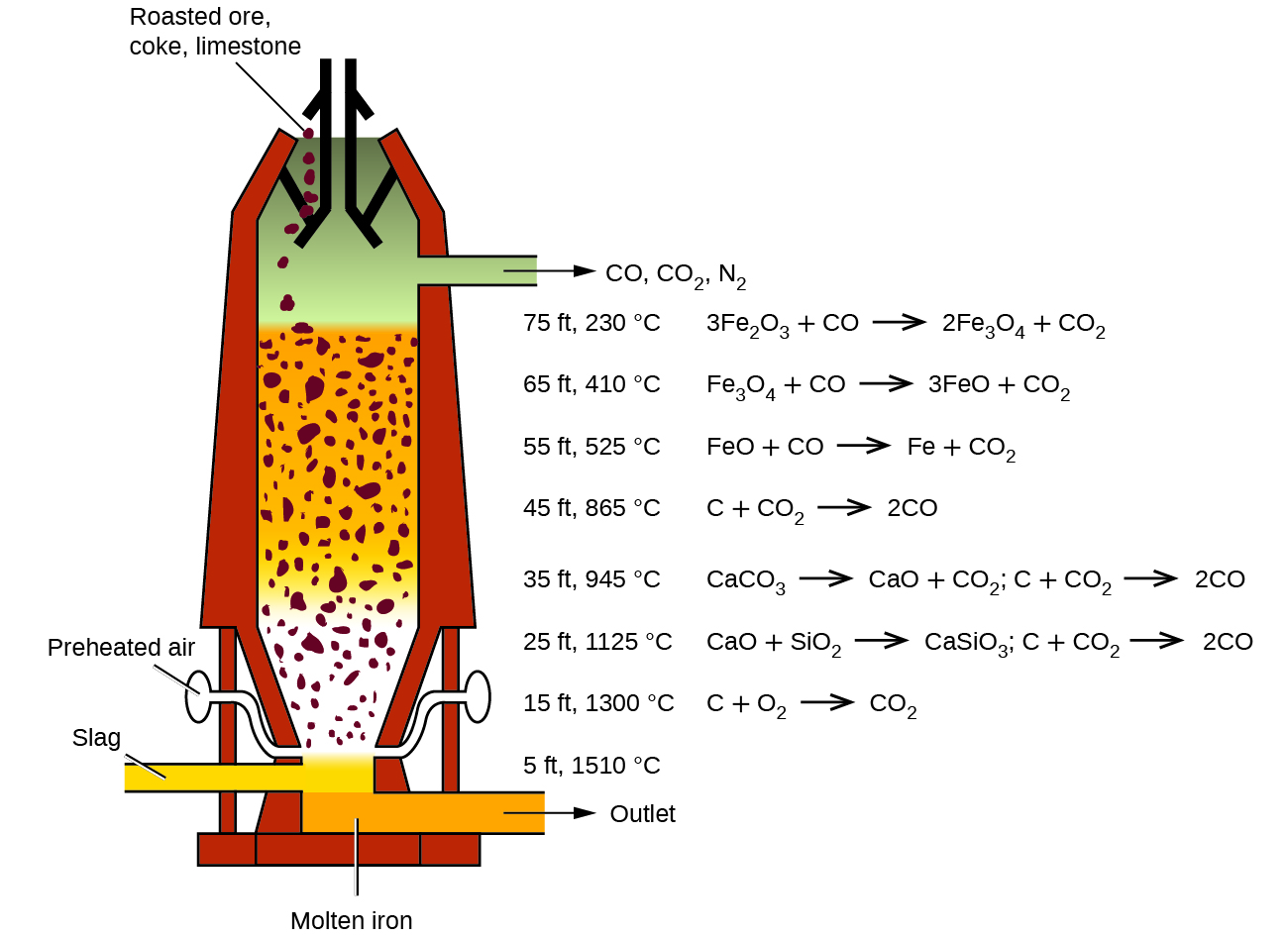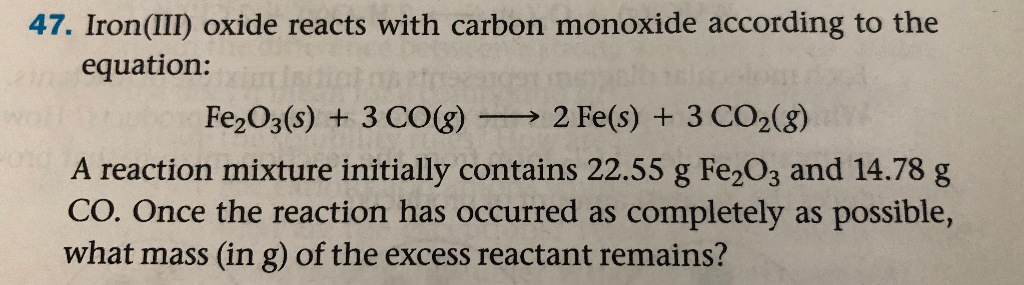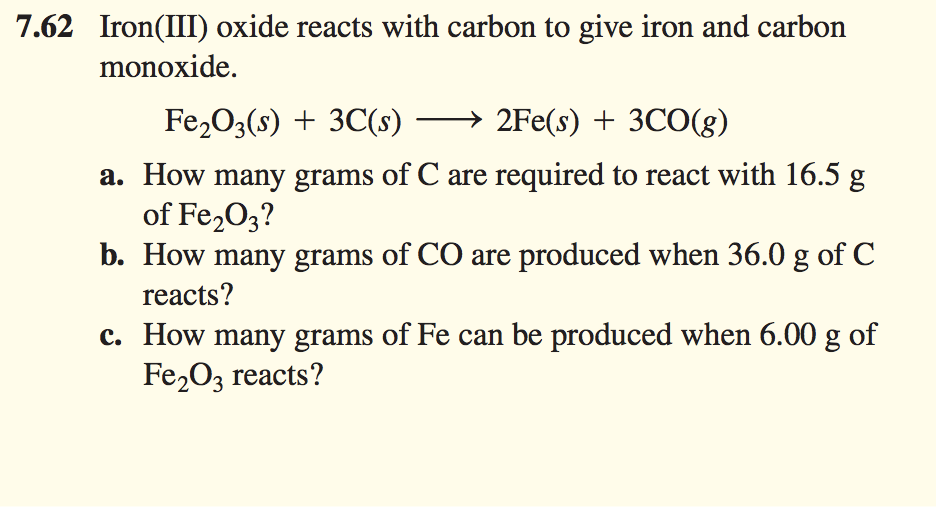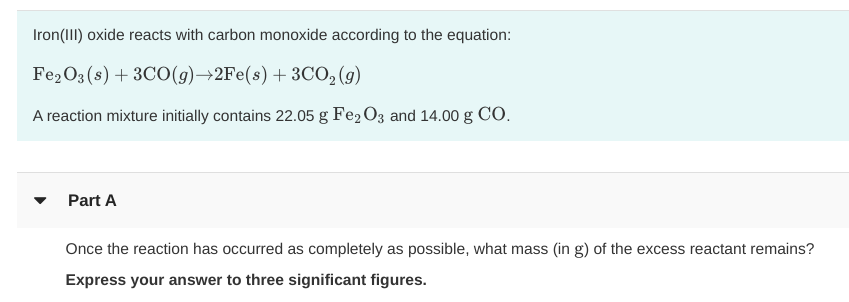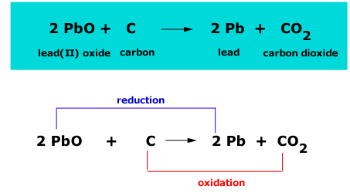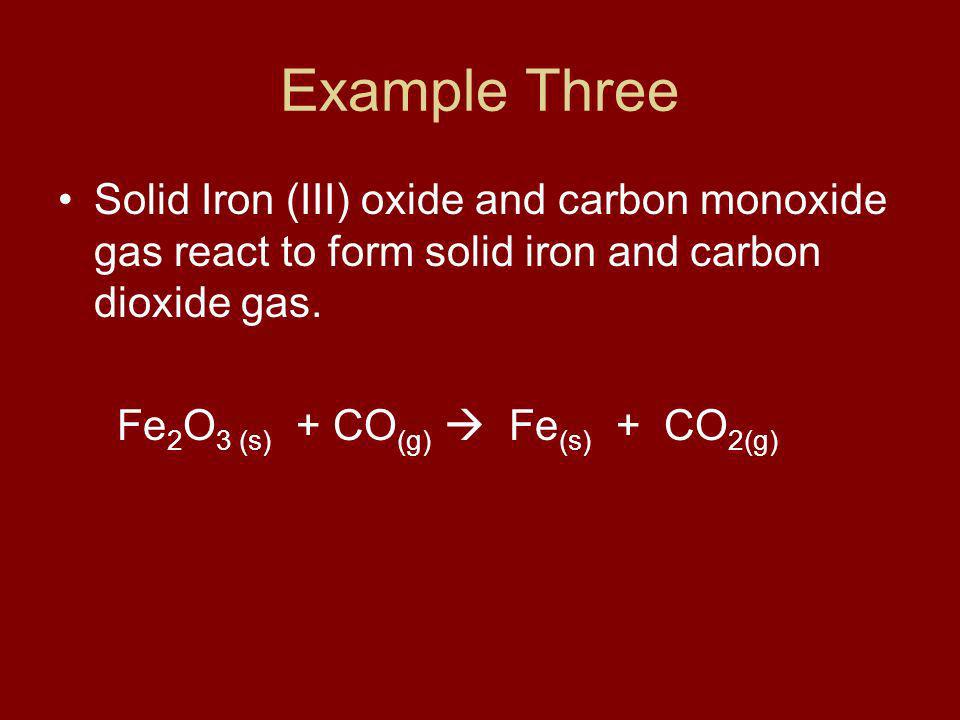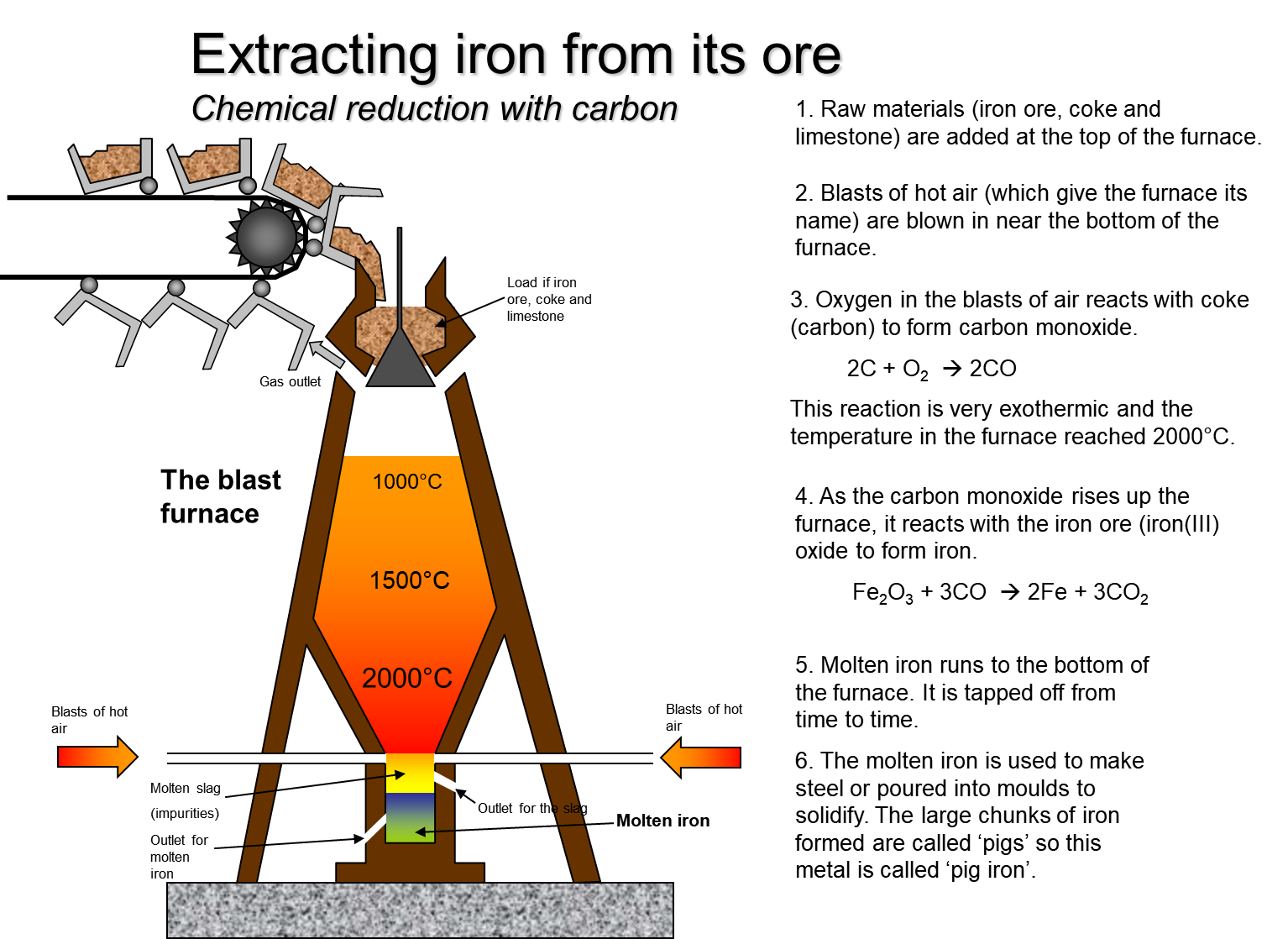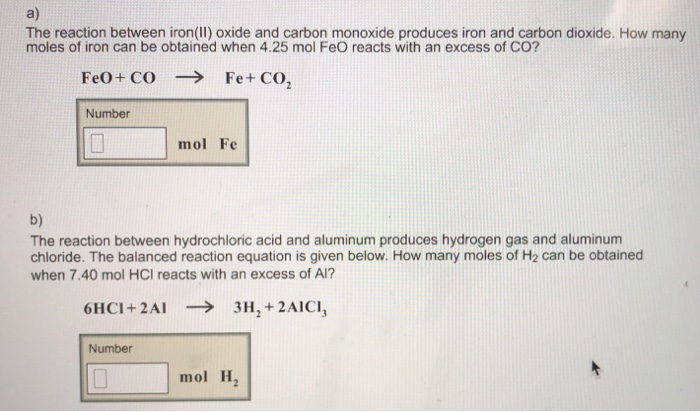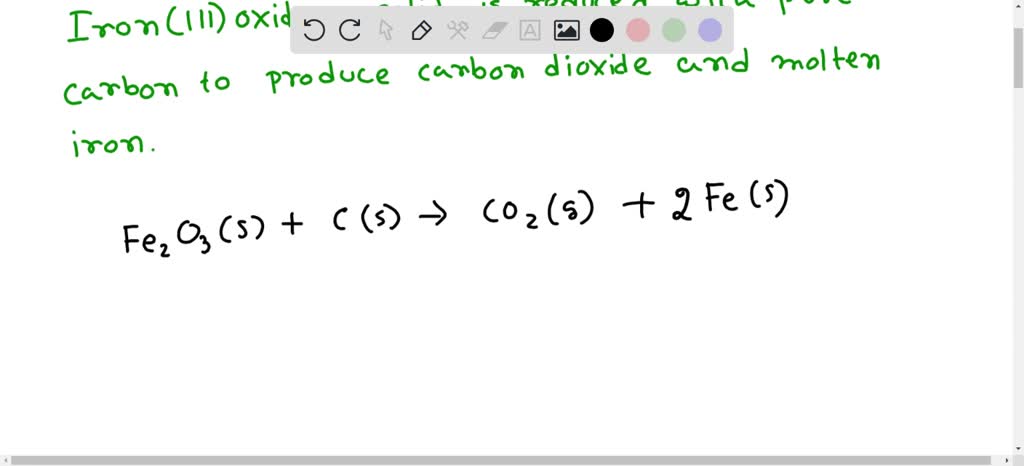
SOLVED: The balanced equation for the reaction occurring when iron(iii) oxide, a solid, is reduced with pure carbon to produce carbon dioxide and molten iron is
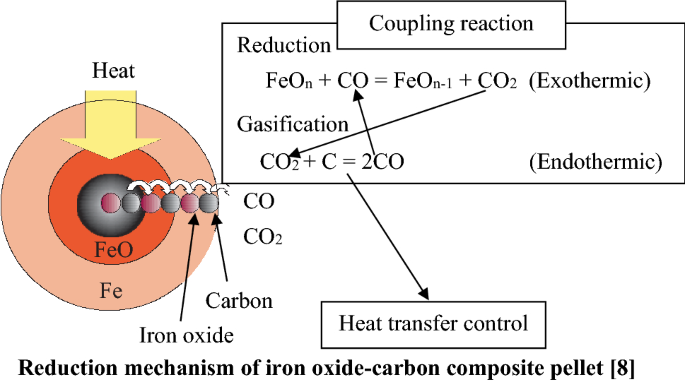
Review on Reduction Kinetics of Iron Ore–Coal Composite Pellet in Alternative and Sustainable Ironmaking | SpringerLink
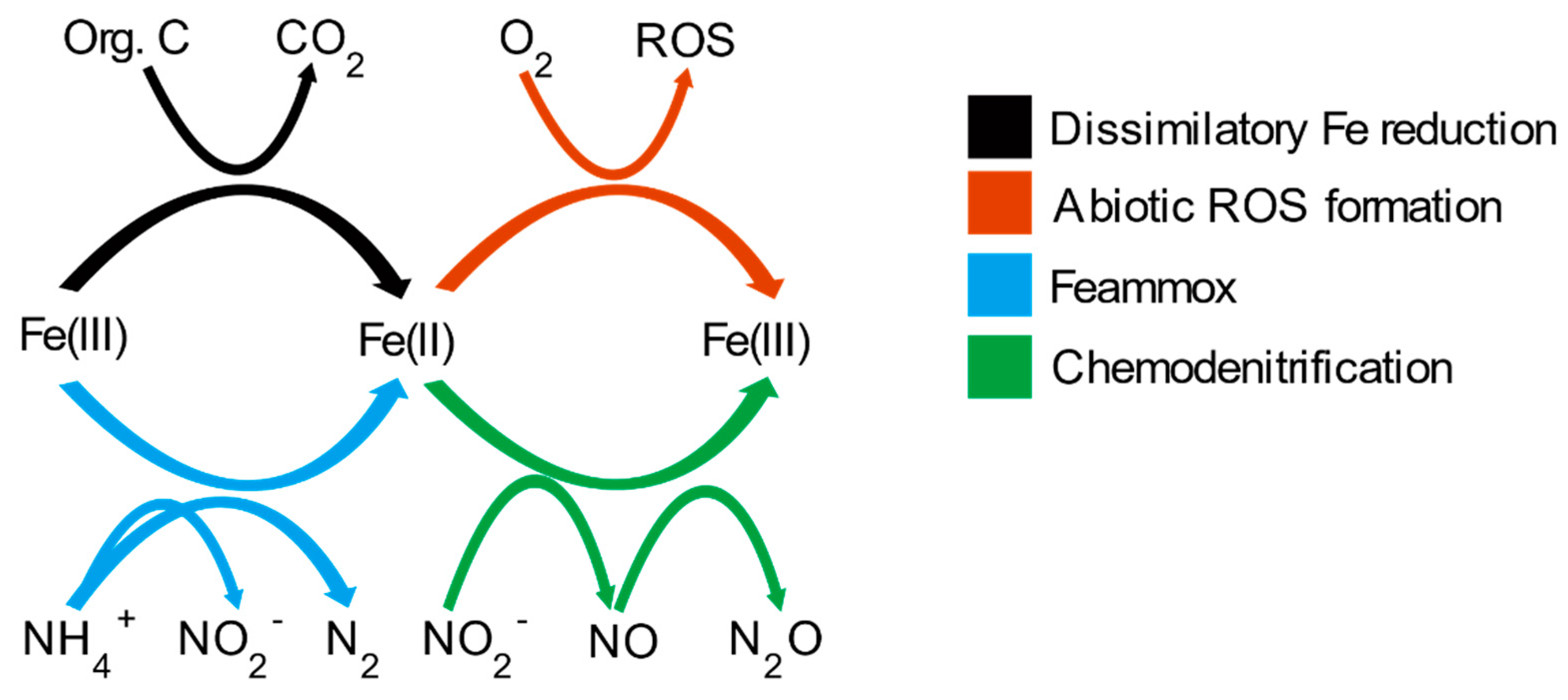
Soil Systems | Free Full-Text | Iron Redox Reactions Can Drive Microtopographic Variation in Upland Soil Carbon Dioxide and Nitrous Oxide Emissions
Iron (III) oxide reacts with carbon monoxide to form solid iron and carbon dioxide. What mass (in grams) of carbon dioxide is produced from 12.4 g of iron (III) oxide? - Quora