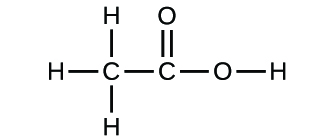
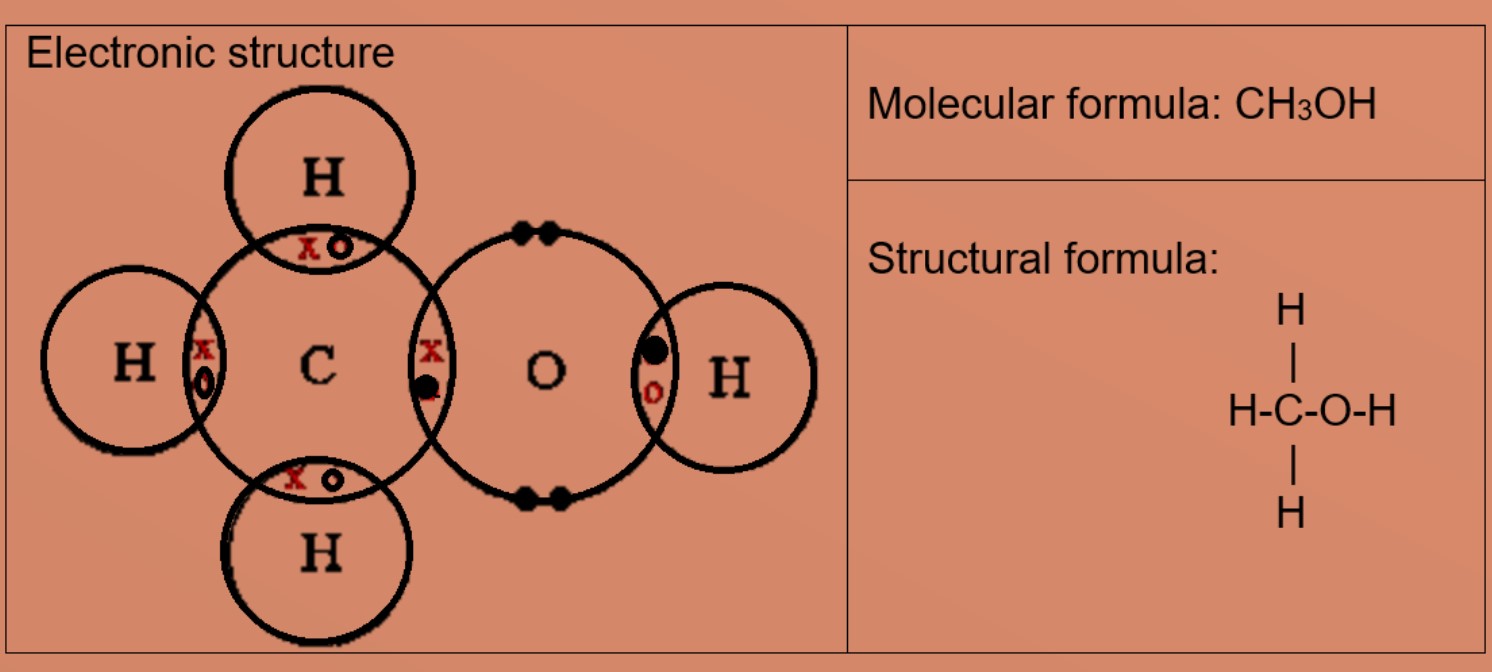
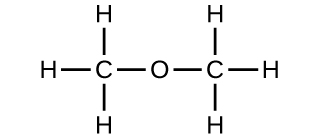
A compound containing only carbon, hydrogen and oxygen was analyzed and found to contain 3.25 % hydrogen and 19.36 % carbon. What is the empricial formula of the compound? | Homework.Study.com

An organic compound contains carbon, hydrogen and oxygen. Its elemental analysis gave C = 38.71% and H, 9.67% and O = 51.62% . The empirical formula of the compound would be:

SOLVED:A compound that contains only carbon, hydrogen, and oxygen is 48.64% C and 8.16% H by mass. What is the empirical formula of this substance?

An organic compound contains carbon, hydrogen and oxygen. If the ratio percentage of C and H is ... - YouTube

Carbon, hydrogen, oxygen, nitrogen, chlorine, and tin atoms are shown... | Download Scientific Diagram

A compound containing carbon, hydrogen and oxygen gas the following analytical data.C=40%,H=6.67% . - Brainly.in

An organic compound contains carbon, hydrogen and oxygen. Its elemental analysis gave`C,38.71% - YouTube
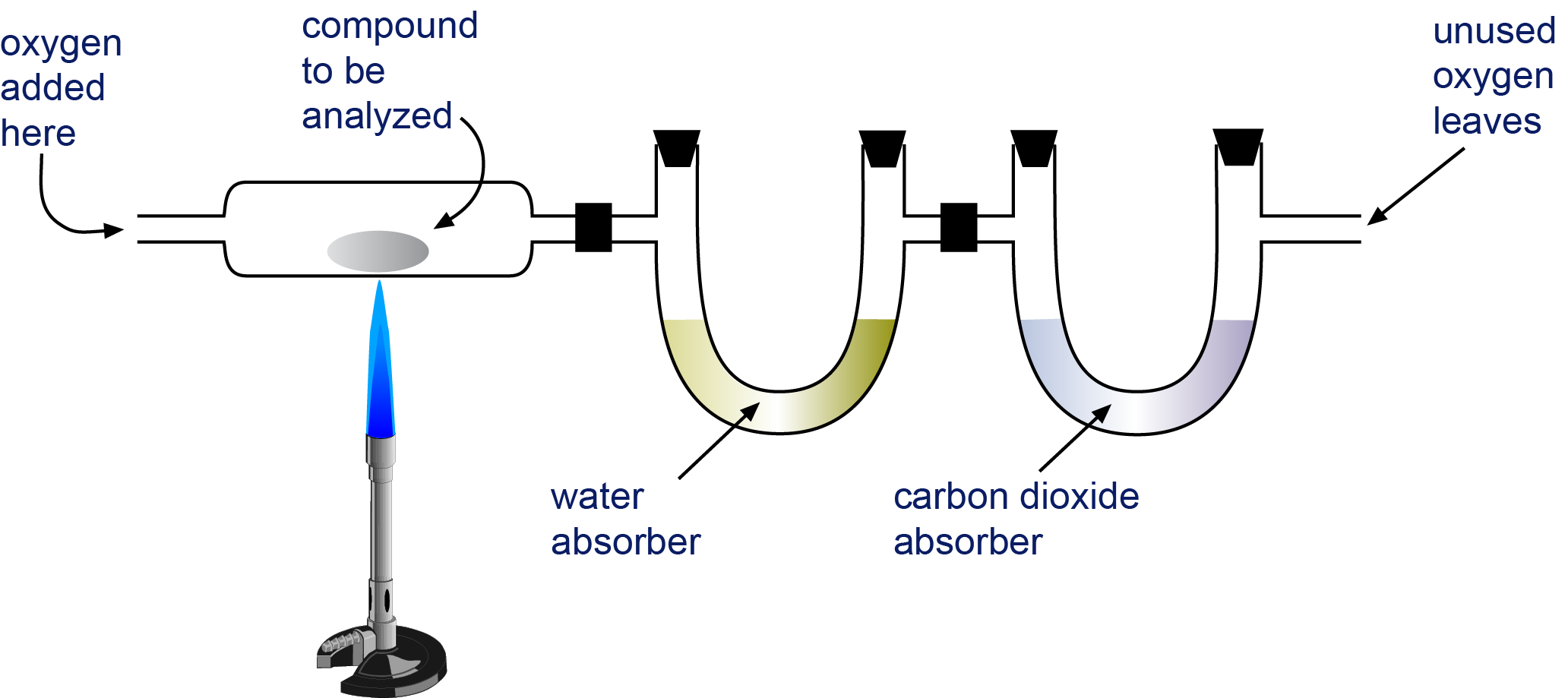
Empirical and molecular formulas for compounds that contain only carbon and hydrogen (C a H b ) or carbon, hydrogen, and oxygen (C a H b O c ) can be determined with a process called combustion analysis. The steps for this procedure are

SOLVED: A compound that contains only carbon, hydrogen, and oxygen is 58.8% C and 9.87% by mass. What is the empirical formula of this substance?

3 A compound containing Carbon, Hydrogen and Oxygen gave the following analytical data: Carbon=40 0% , Hydrogen =6 67% - Chemistry - Some Basic Concepts of Chemistry - 12771131 | Meritnation.com