Carbon monoxide was first prepared by Lasson in 1776,by heating zinc oxide with wood charcoal.It was mistaken for hydrogen, because it burnt with a pale. - ppt download

Reduction Oxidation Chapter 14 and. Oxygen is the most abundant element on Earth and is involved in many of the most important chemical reactions in our. - ppt download

Following is used as a reducing agent in metallurgy i.e. extraction of metals in blast furnace. ... - YouTube

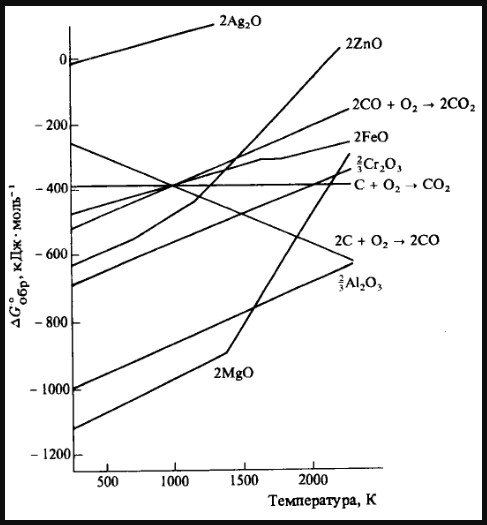
Zinc oxide can be reduced to zinc by using carbon monoxide, but aluminium oxide cannot be reduced by a reducing agent. Explain why?

The Conversion of Carbon Monoxide and Carbon Dioxide by Nitrogenases - Oehlmann - 2022 - ChemBioChem - Wiley Online Library




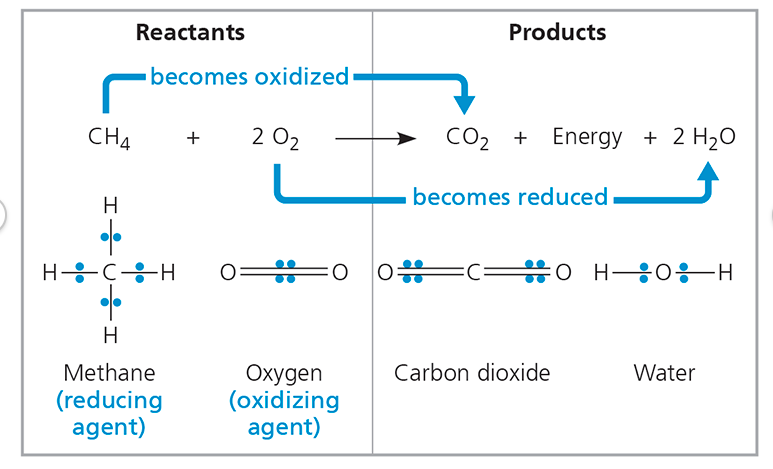






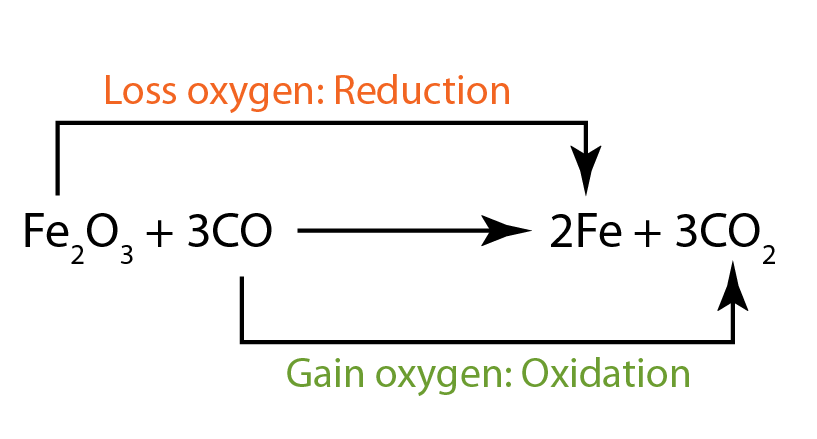
:max_bytes(150000):strip_icc()/what-is-carbon-monoxide-Final-27d97041ac95424c9af73c0dd25adcad.jpg)