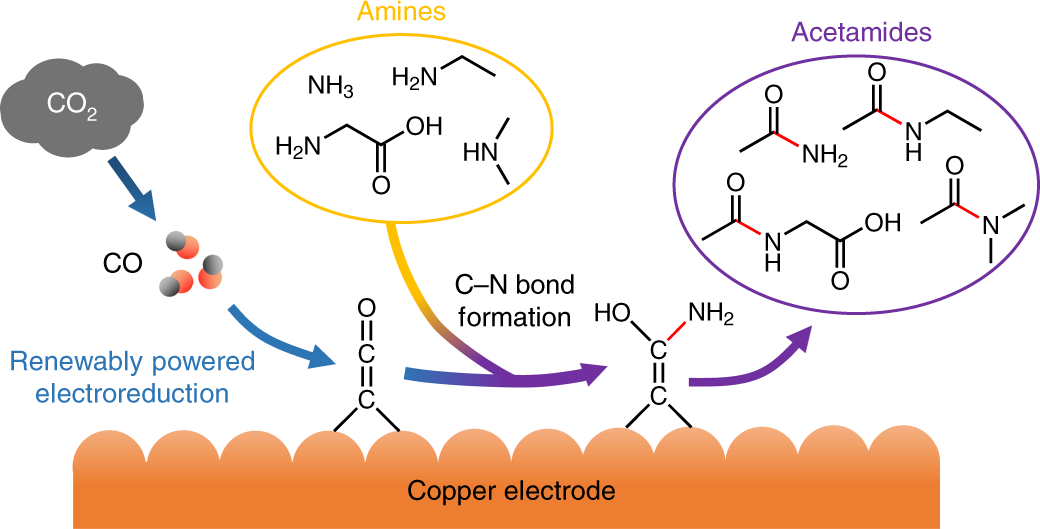
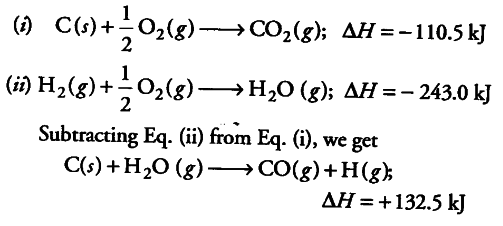The heat of formation of CO(g) and CO2(g) are Δ H = - 110 and Δ H = - 393kJmmol^-1 respectively. What is the heat of reaction (Δ H) (in kJ mol^-1 )

Phosgene formation via carbon monoxide and dichlorine reaction over an activated carbon catalyst: Towards a reaction model - ScienceDirect

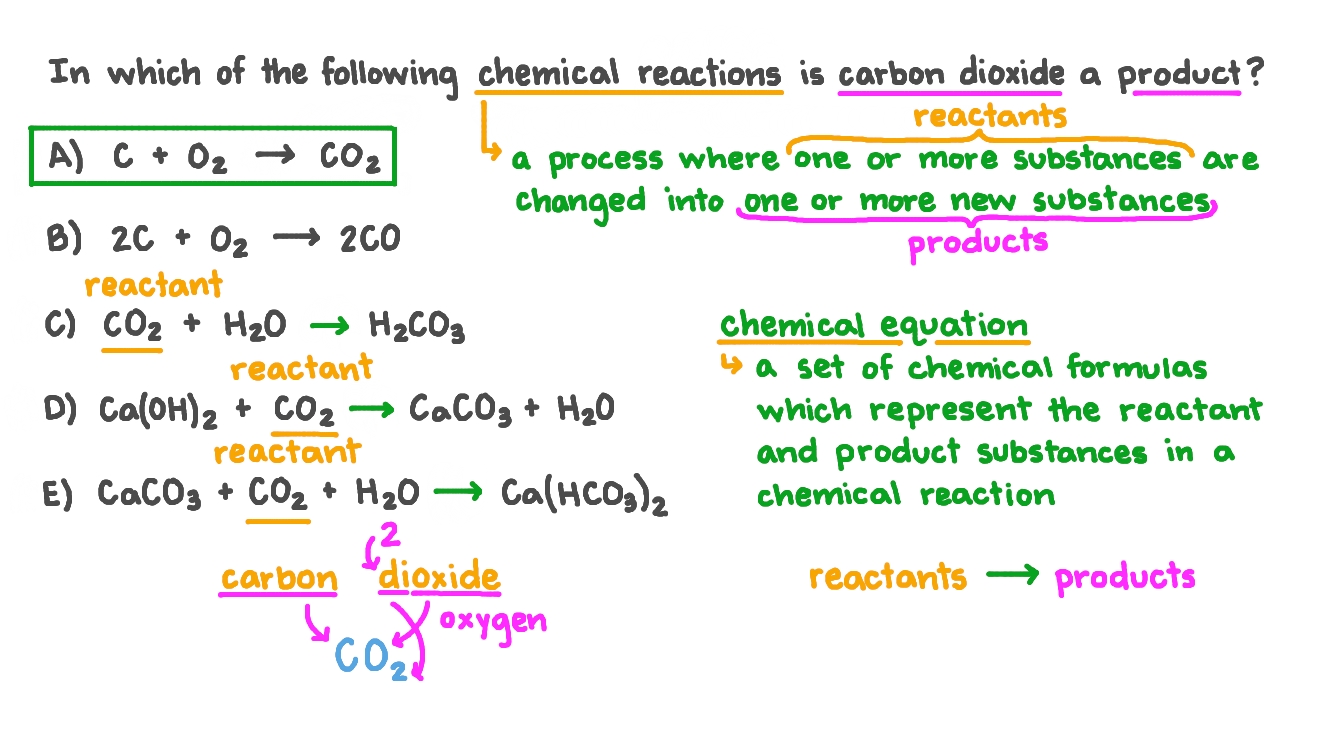
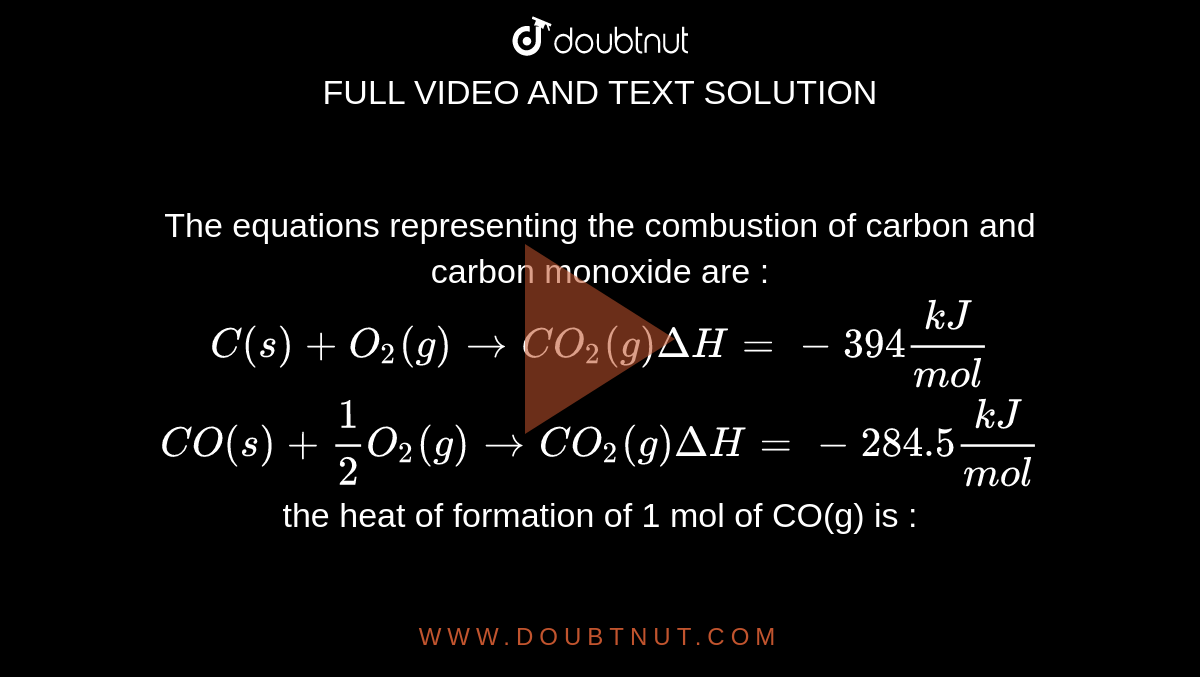
The enthalpy of combustion of carbon and carbon monoxide are - 393.5 and - 283 kJ/mol respectively. The enthalpy of formation of carbon monoxide per mole is:

Transition metal–free ketene formation from carbon monoxide through isolable ketenyl anions | Science
Carbon Formation from Carbon Monoxide-Hydrogen Mixtures over Iron Catalysts.I. Properties of Carbon Formed | The Journal of Physical Chemistry

The energy levels of carbon monoxide molecules and the formation of... | Download Scientific Diagram
Formation of carbon monoxide and carbon dioxide molecules: (1) empty... | Download Scientific Diagram

Reductive Cleavage of the CO Molecule by a Reactive Vicinal Frustrated PH/BH Lewis Pair | Journal of the American Chemical Society

The heat of formations of CO(g) and CO2 (g) are - 26.4 kcal and - 94.0 kcal receptively, The heat of combination of carbon monoxide will be