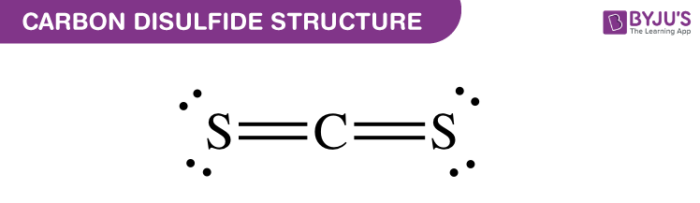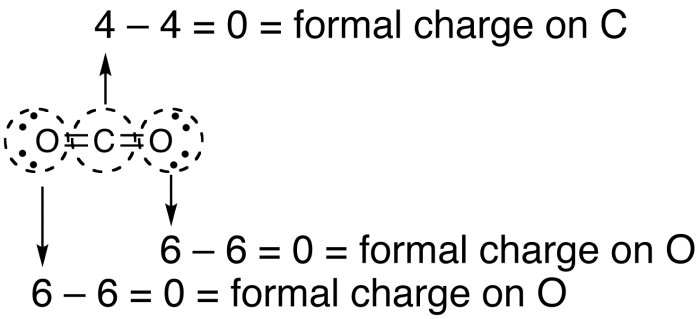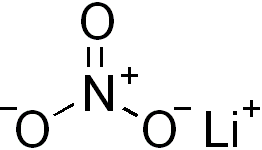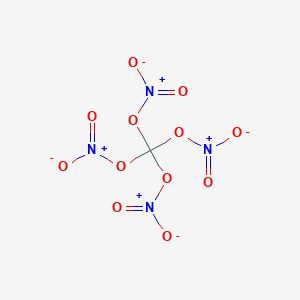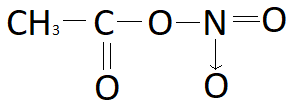
Acetyl nitrate has the structure:A. \n \n \n \n \n B.\n \n \n \n \n C.\n \n \n \n \n D. \n \n \n \n \n
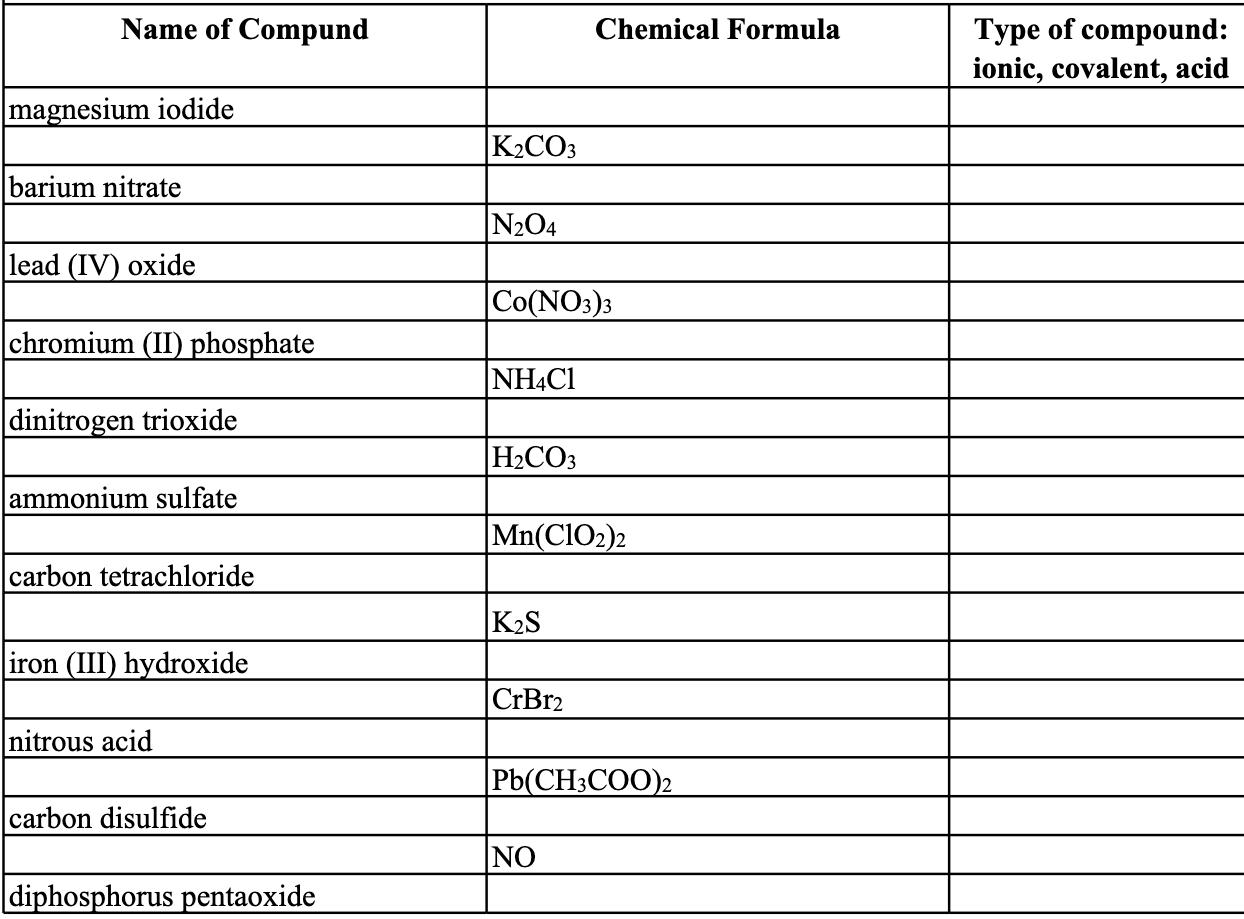
SOLVED: Write formulas for each of the following compounds: a. potassium hydroxide b. calcium nitrate c. sodium carbonate d. carbon tetrachloride e. magnesium bromide

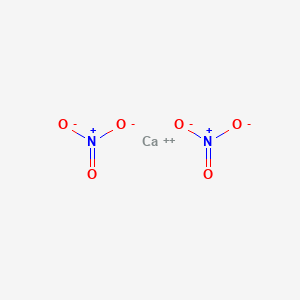
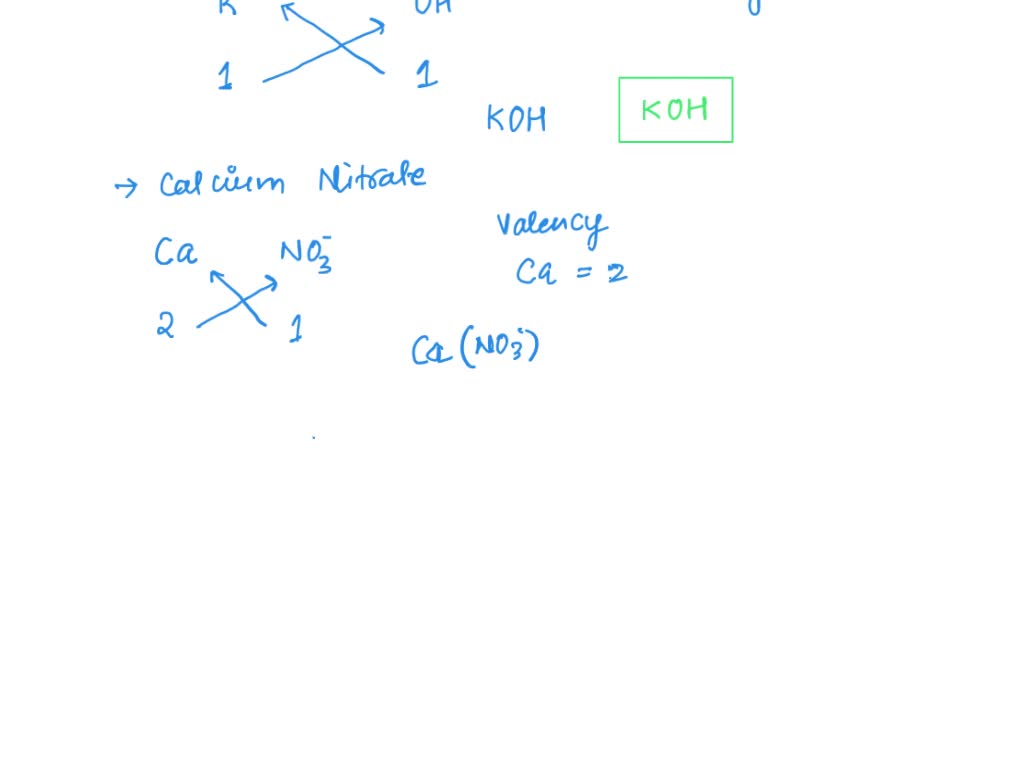
SOLVED: name formula Calcium hydroxide Ca(OH)2 Carbon dioxide COz Calcium carbonate CaCO; Potassium nitrate KNO; Calcium nitrate Ca(NO3)2 Calcium sulfate CaSO4 Ammonium carbonate (NH4)COz Barium hydroxide Ba(OH)2 Ammonium sulfate (NH4JSO4 Barium sulfate
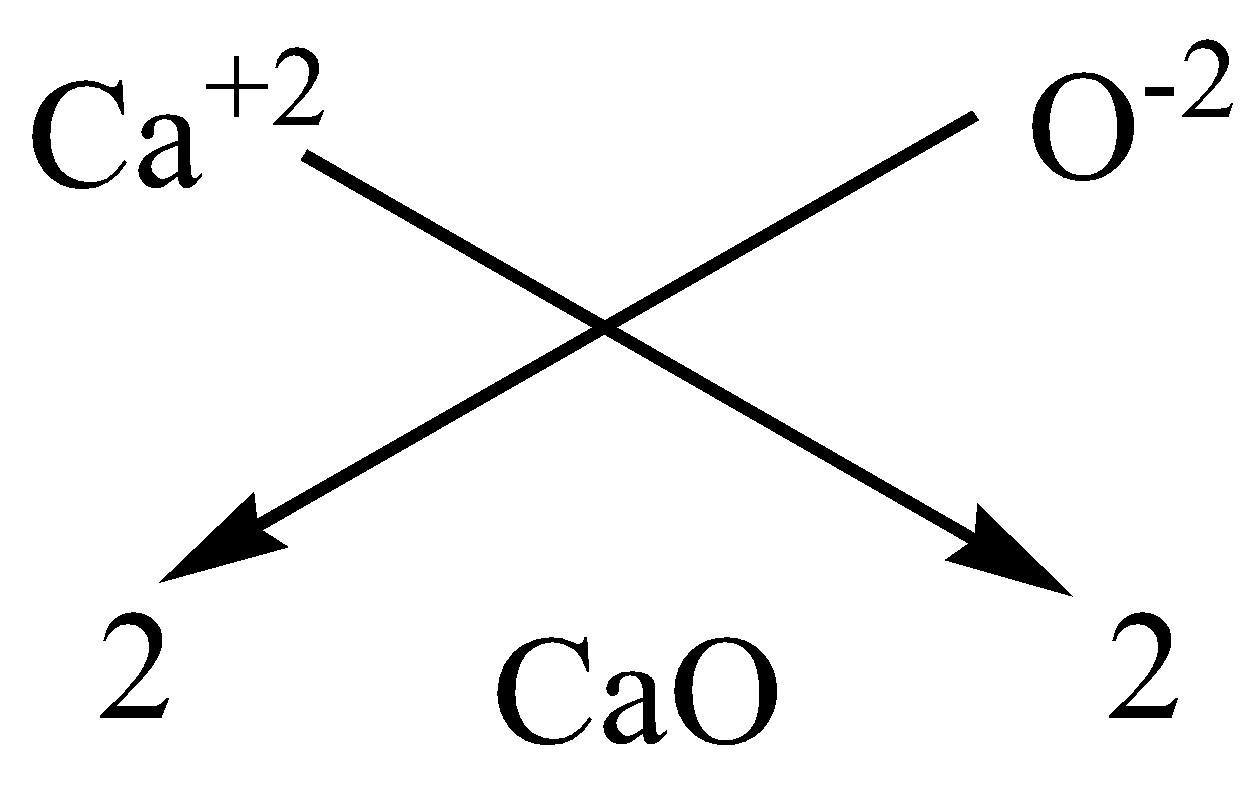
Write the chemical formulae of the following by criss-cross method:A.Magnesium chlorideB.Calcium oxideC.Copper nitrateD.Aluminium chlorideE.Potassium nitrate

Sensors | Free Full-Text | Graphitic Carbon Nitride: A Highly Electroactive Nanomaterial for Environmental and Clinical Sensing

Surkaj Pharmaceuticals Incorporation - Molecular Formula for Common Chemicals A molecular formula is an expression of the number and type of atoms that are present in a single molecule of a substance.

Q3 Give a balanced equation for the following conversions In one or two steps 1 Coke to water gas 2 ...
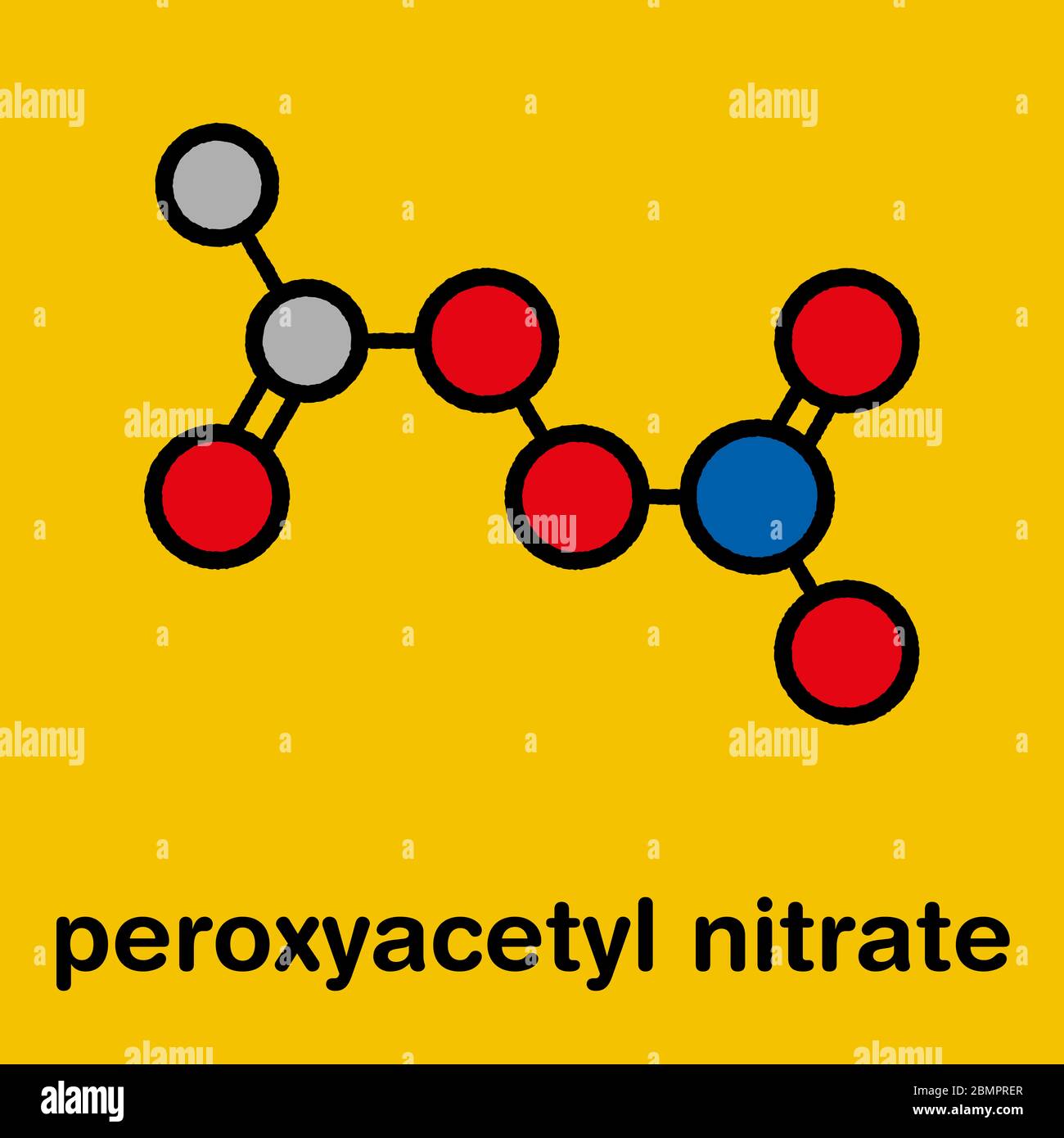
Peroxyacetyl nitrate (PAN) pollutant molecule. Secondary pollutant, found in photochemical smog. Further decomposes into peroxyethanol radical and nitrogen dioxide. Stylized skeletal formula (chemical structure): Atoms are shown as color-coded circles ...

i) A gas of mass 32 gms has volume of 20 litres at S.T.P. Calculate the gram molecular weight of the gas.(ii) How much Calcium oxide is formed when 82 g of

NAMING RULES 1)Determine type of bond 2) COVALENT non-metal + non-metal TYPE I IONIC non-metal + (col. I,II, Al) TYPE II IONIC non-metal + transition. - ppt download

![Barium Nitrate [Ba(NO3)2] Molecular Weight Calculation - Laboratory Notes Barium Nitrate [Ba(NO3)2] Molecular Weight Calculation - Laboratory Notes](https://www.laboratorynotes.com/wp-content/uploads/2023/03/barium-nitrate-molecular-weight-calculation-300x197.jpg)