
Hot carbon reacts with steam to produce an equimolar mixture of CO(g) and H2(g) known as water gas. What is the energy released as water gas is used as fuel? CO(g) +

1111 Chemistry 132 NT I never let my schooling get in the way of my education. Mark Twain. - ppt download
![PDF] Thermodynamic analysis of Glycerol Steam Reforming for hydrogen production with in situ hydrogen and carbon dioxide separation | Semantic Scholar PDF] Thermodynamic analysis of Glycerol Steam Reforming for hydrogen production with in situ hydrogen and carbon dioxide separation | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/63d77a6e33c8b41fb11c2333453dd9c6776ef3aa/19-Table1-1.png)
PDF] Thermodynamic analysis of Glycerol Steam Reforming for hydrogen production with in situ hydrogen and carbon dioxide separation | Semantic Scholar
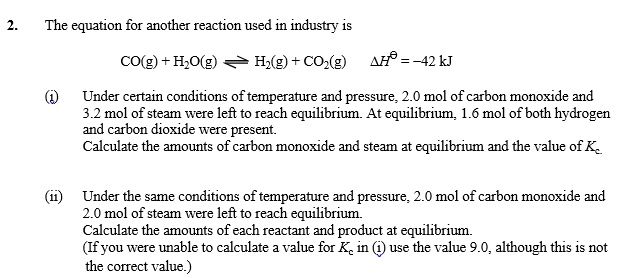
SOLVED: The equation for another reaction used in industry is cO(g) + H,Olg) Hz(g) + CO-(g) AH = -42 kJ Under certain conditions of temperature and pressure; 2.0 mol of carbon monoxide

Reactivity of carbon toward steam by different reaction temperatures... | Download Scientific Diagram
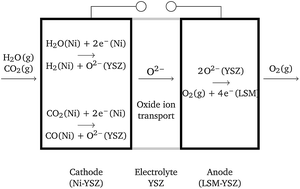
Thermodynamic analysis of high temperature steam and carbon dioxide systems in solid oxide cells - Sustainable Energy & Fuels (RSC Publishing)
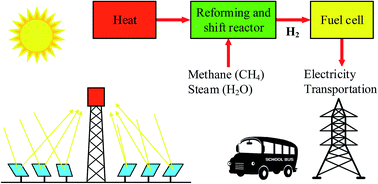
Process analysis of solar steam reforming of methane for producing low- carbon hydrogen - RSC Advances (RSC Publishing)






![The key reactions of steam reforming process [200]. | Download Table The key reactions of steam reforming process [200]. | Download Table](https://www.researchgate.net/publication/330701158/figure/tbl3/AS:720247648706562@1548731829841/The-key-reactions-of-steam-reforming-process-200.png)


