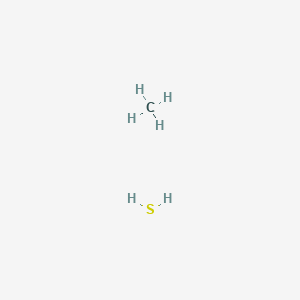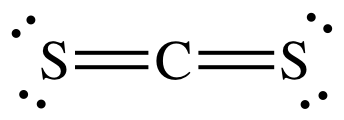theoretical chemistry - Carbon-Sulfur Bond Lengths; Resonance Effects (Or Lack Thereof) - Chemistry Stack Exchange
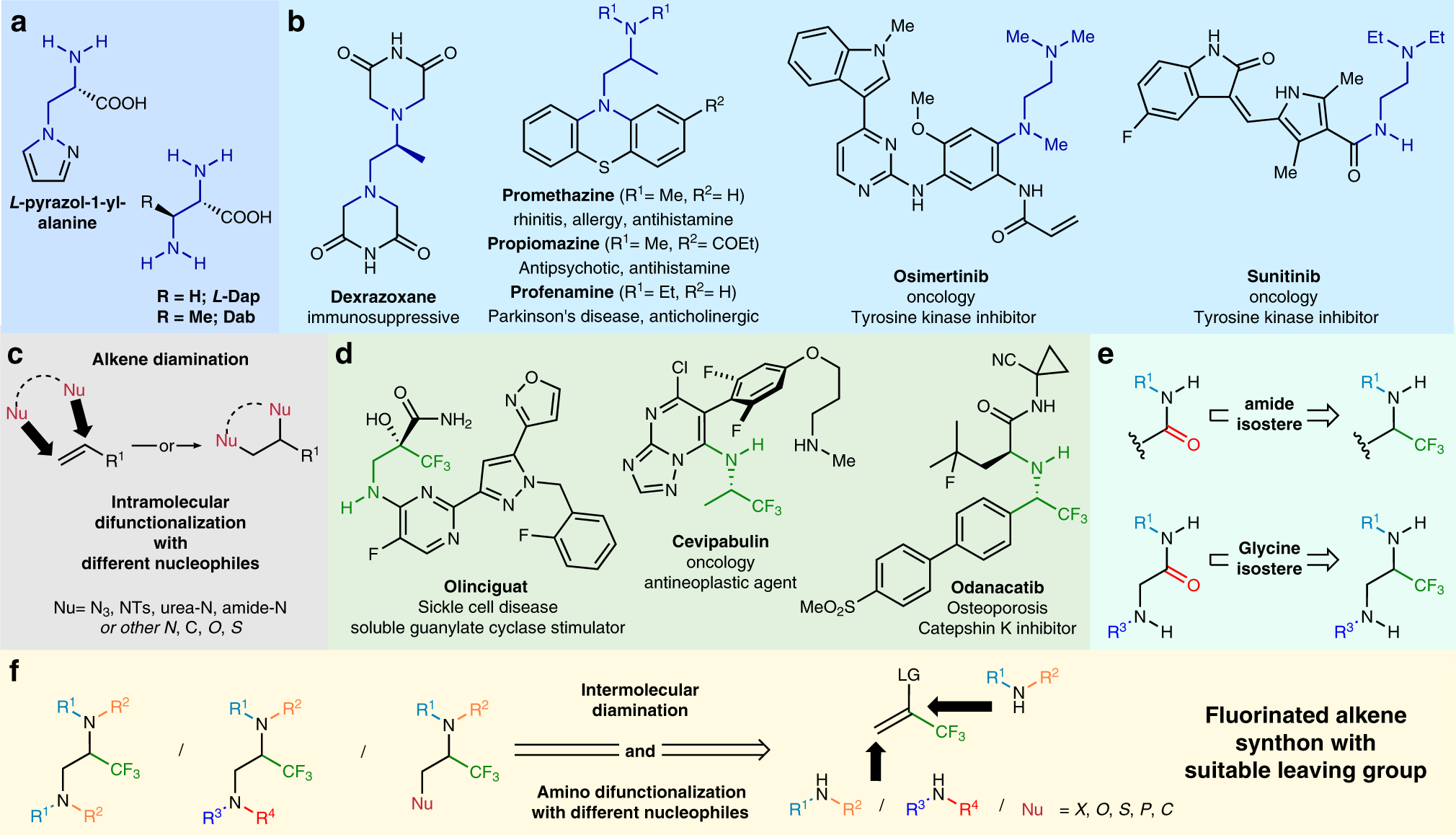
Vicinal difunctionalization of carbon–carbon double bond for the platform synthesis of trifluoroalkyl amines | Nature Communications

7.70 | Calculate the bond energy of the carbon-sulfur double bond in CS2 from the standard enthalpy - YouTube

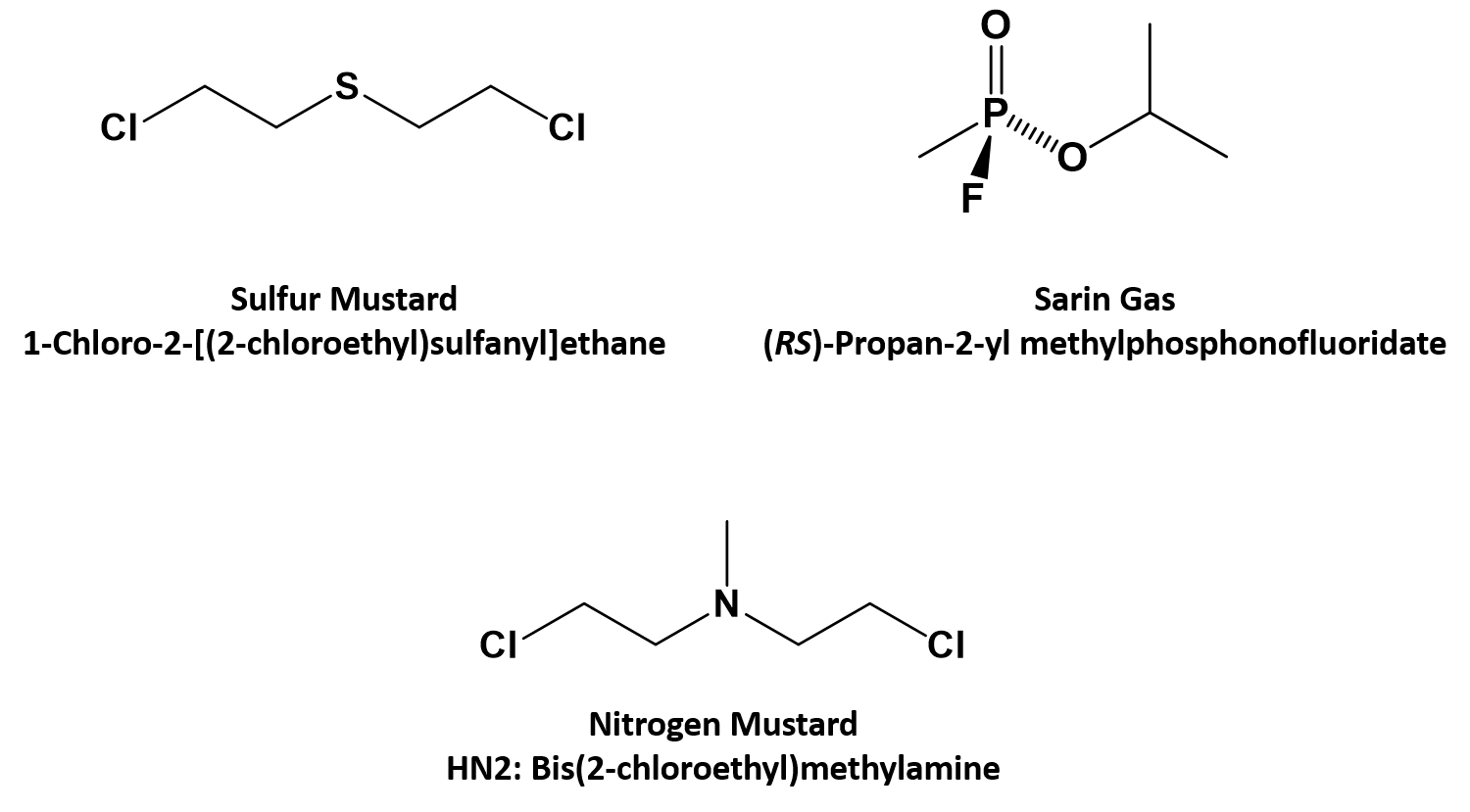
Comparison of carbon-sulfur and carbon-amine bond in therapeutic drug: 4β-S-aromatic heterocyclic podophyllum derivatives display antitumor activity | Scientific Reports

theoretical chemistry - Carbon-Sulfur Bond Lengths; Resonance Effects (Or Lack Thereof) - Chemistry Stack Exchange

Carbon–sulfur bond formation via photochemical strategies: An efficient method for the synthesis of sulfur-containing compounds - ScienceDirect
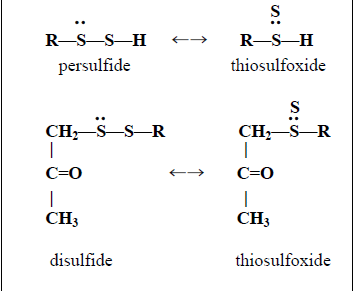
Molecules | Free Full-Text | Thiosulfoxide (Sulfane) Sulfur: New Chemistry and New Regulatory Roles in Biology

Chapter 3: Alkanes and Their Stereochemistry. Functional Groups Functional groups are a group of atoms that has similar characteristic chemical behavior. - ppt download

Methanethiol, CH_3SH, has a substantial dipole moment (mu = 1.52) even though carbon and sulfur have identical electronegativities. Explain. | Homework.Study.com
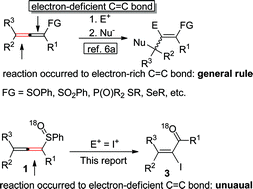
Cleavage of oxygen–sulfur double bonds and carbon–sulfur bonds: unusual highly selective electrophilic addition of allenylic sulfoxides - Chemical Science (RSC Publishing)
![Competitive Carbon−Sulfur vs Carbon−Carbon Bond Activation of 2-Cyanothiophene with [Ni(dippe)H]2 | Journal of the American Chemical Society Competitive Carbon−Sulfur vs Carbon−Carbon Bond Activation of 2-Cyanothiophene with [Ni(dippe)H]2 | Journal of the American Chemical Society](https://pubs.acs.org/cms/10.1021/ja104158h/asset/images/ja104158h.social.jpeg_v03)
Competitive Carbon−Sulfur vs Carbon−Carbon Bond Activation of 2-Cyanothiophene with [Ni(dippe)H]2 | Journal of the American Chemical Society