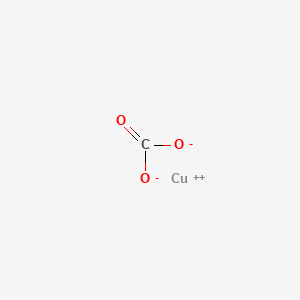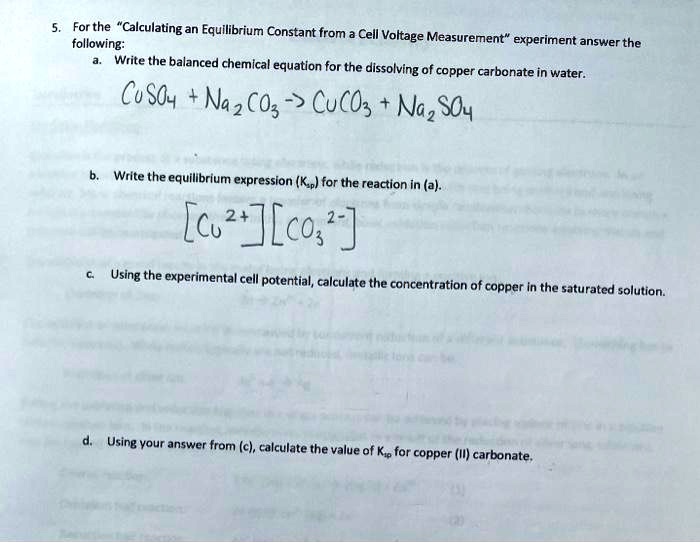
SOLVED: For the "Calculating an Equilibrium Constant from following: Cell Voltage Measurement" experiment answer the Write the balanced chemical equation for the dissolving of = copper carbonate in water: CoSOy NazC C0;

SOLVED: Copper carbonate (CuCO3) reacts with hydrochloric acid (HCl) according to this equation: CuCO3(s) + 2HCl(aq) → CuCl2(aq) + H2O(l) + CO2(g). Which statement correctly describes the substances in this reaction? A.

Negative ion Formula in compoundcharge Oxide Hydroxide Nitrate NO 3 -1 Sulphate Carbonate. - ppt download