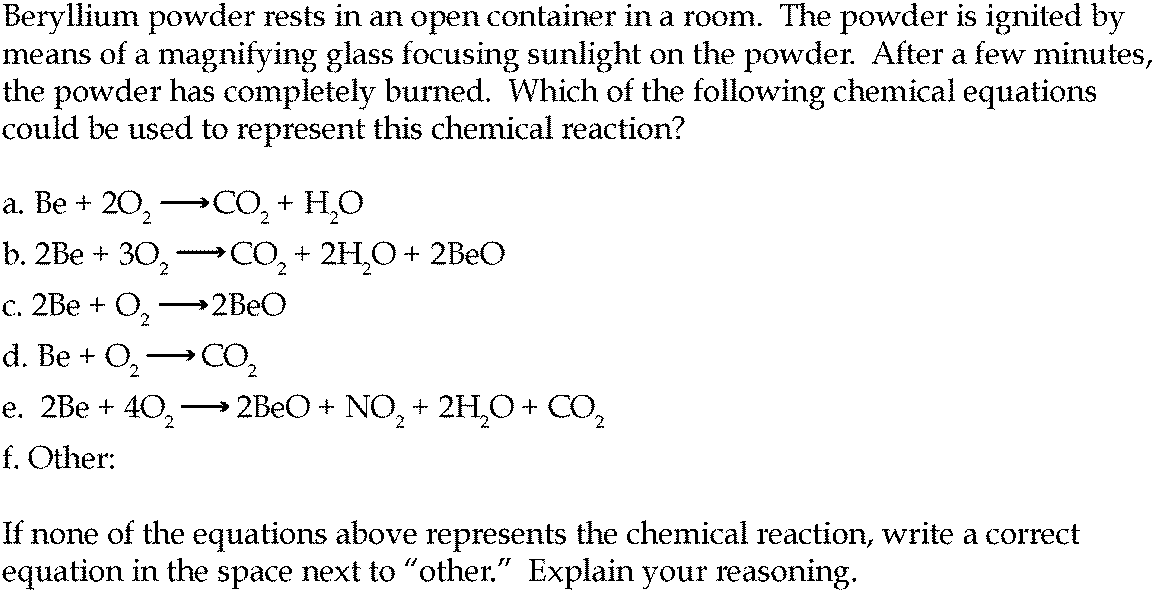
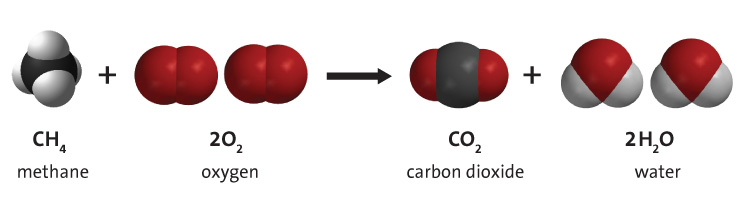
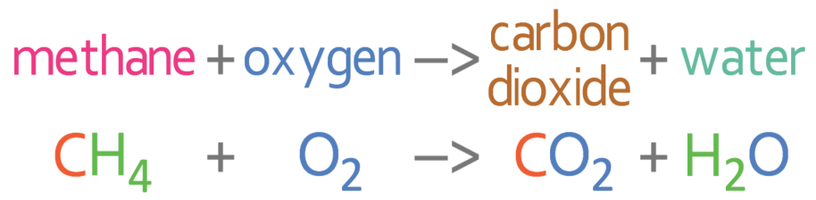Carbon Dioxide Separation from Flue Gases: A Technological Review Emphasizing Reduction in Greenhouse Gas Emissions

Write the balanced chemical equation for combustion of carbon dioxide - Science - Chemical Reactions and Equations - 12555075 | Meritnation.com
20. One mole of carbonundergoes incomplete combustion to produce carbon monoxide. Calculate(AH Δ ) for the formation of CO at 298 KGiven R 8314 JK 1 mol 1

How is the combustion of carbon a redox reaction? I get the oxidation part, but where is the reduction? - Quora
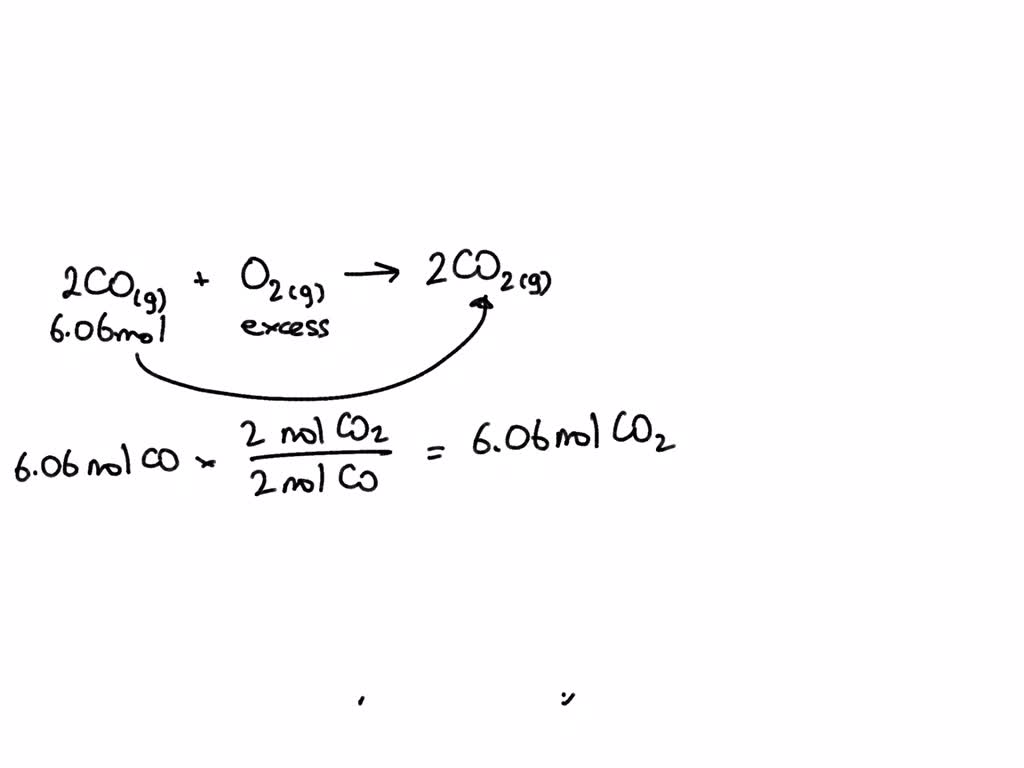
SOLVED: Consider the combustion of carbon monoxide (CO) in oxygen gas: 2CO(g) + O2(g) → 2CO2(g) Calculate the number of moles of CO2 produced if 6.06 moles of CO are reacted with
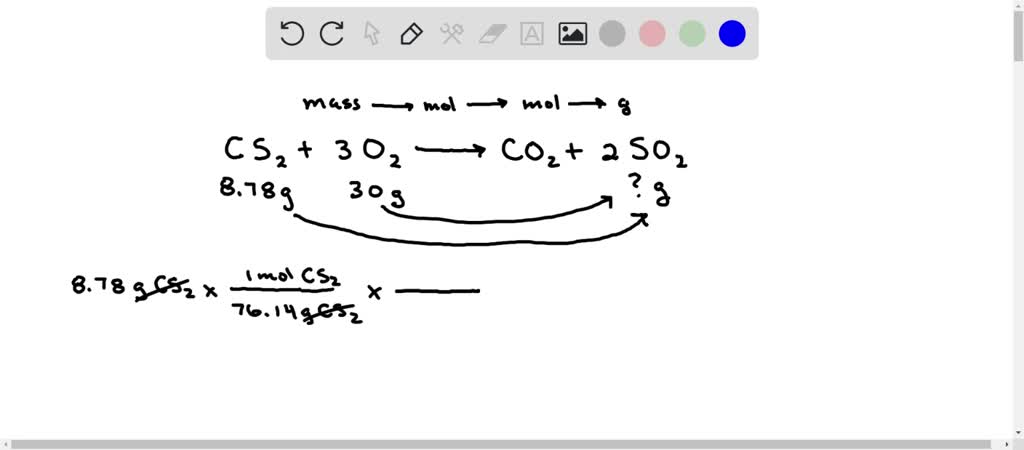
SOLVED: The combustion of carbon disulfide in the presence of excess oxygen yields carbon dioxide and sulfur dioxide according to the following UNBALANCED reaction: CS2 (g) + 3O2 (g) → CO2 (g) +

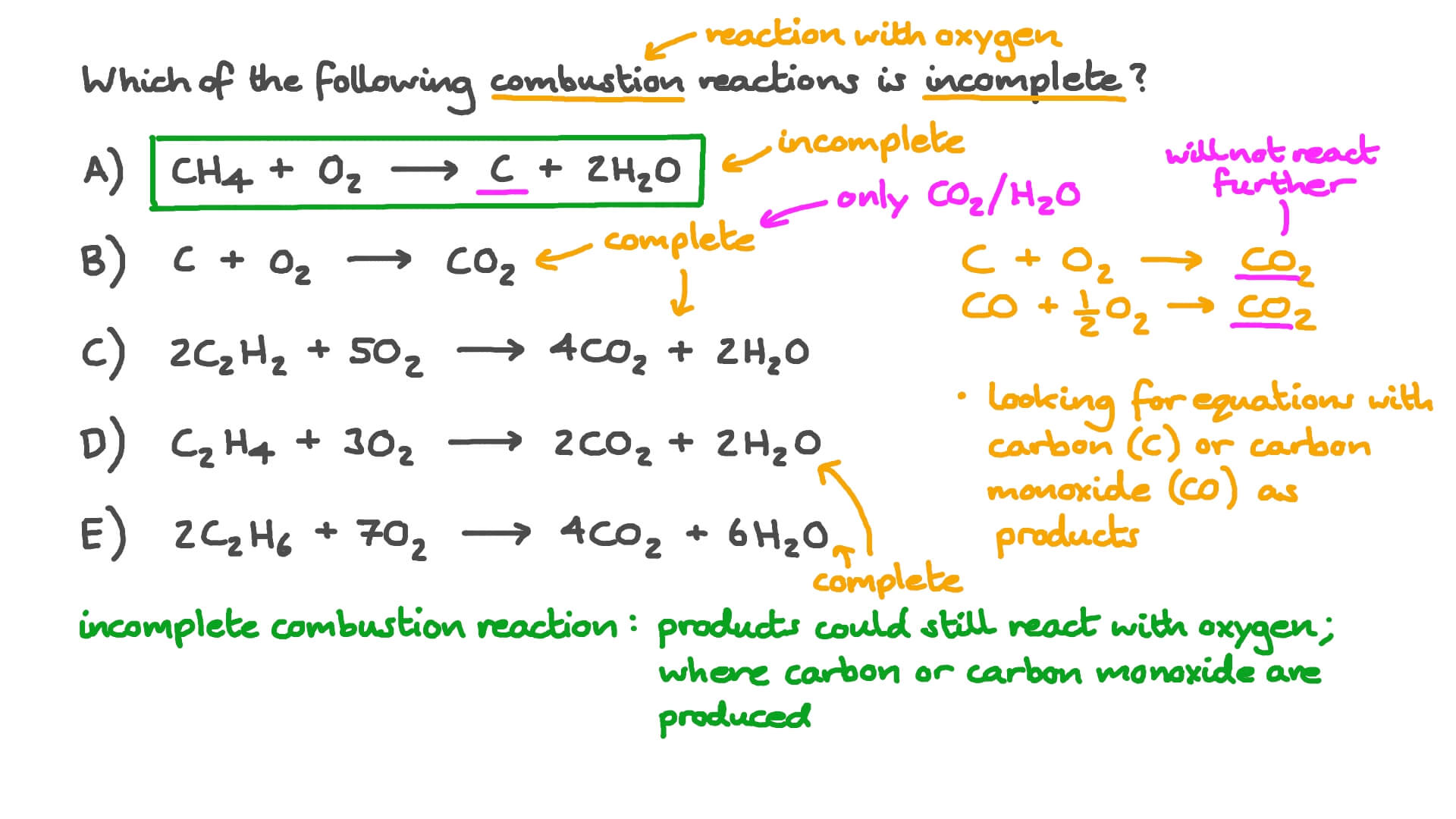
OneClass: The combustion of carbon monoxide is represented by the equation above Determine the value ...
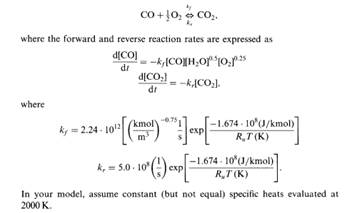
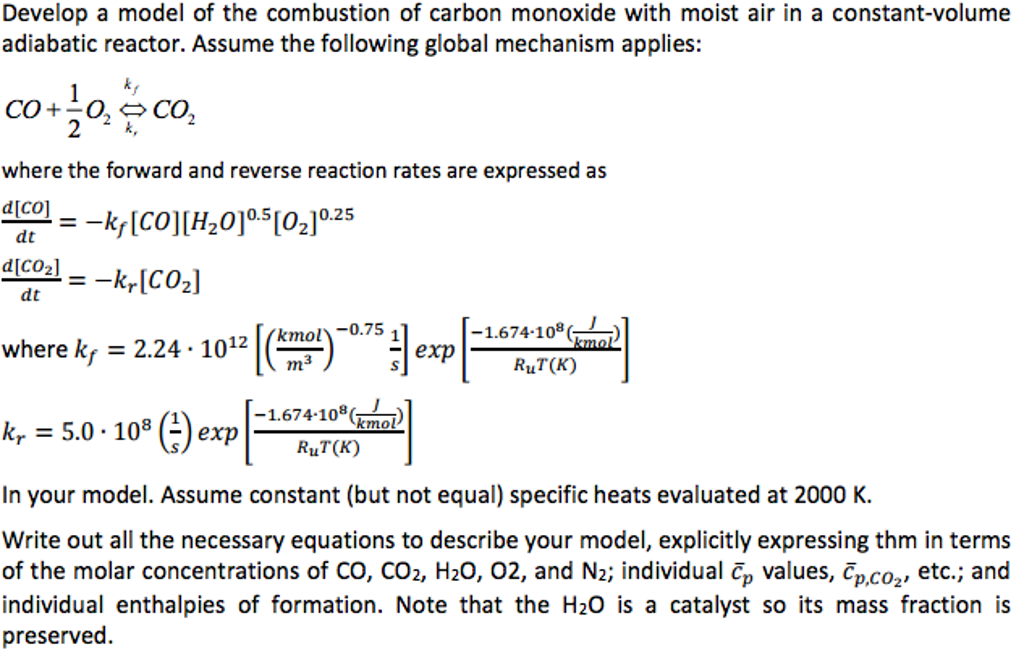
Solved) - Develop a model of the combustion of carbon monoxide with moist... - (3 Answers) | Transtutors

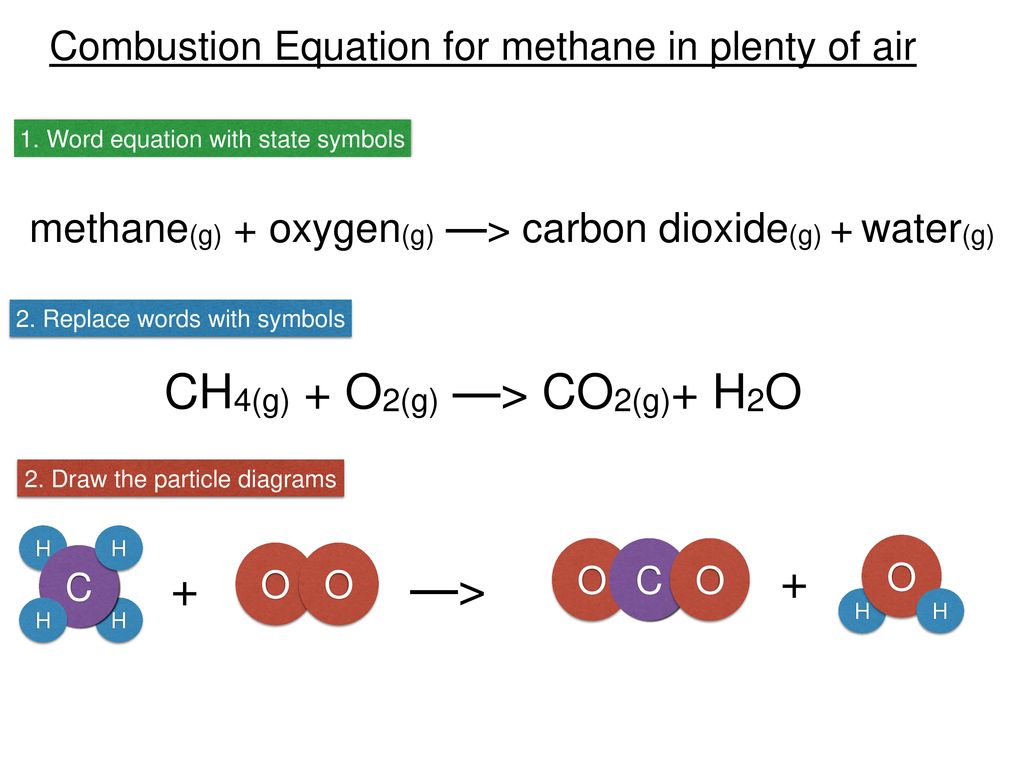
Combustion of hydrocarbons When hydrocarbons are heated in air they react with oxygen forming carbon dioxide or carbon monoxide or carbon, and water. The. - ppt download

The enthalpy of combustion of carbon and carbon monoxide are - 393.5 and - 283 kJ/mol respectively. The enthalpy of formation of carbon monoxide per mole is:

Enthalpy of combustion of carbon to CO2 is - 393.5 KJ/mole. The heat released upon the formation of 35.2g of CO2 from carbon and dioxygen gas is.
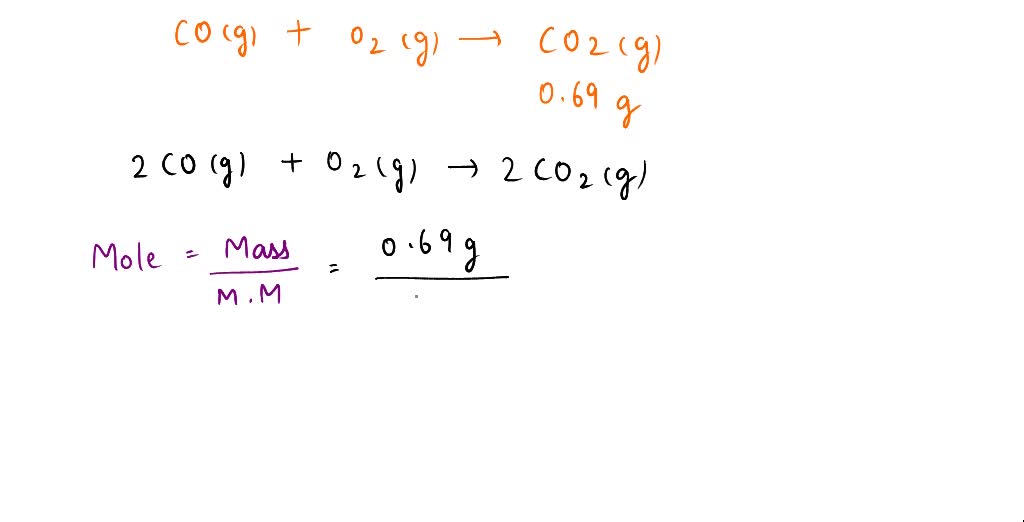
SOLVED: In the combustion of carbon monoxide, what mass of CO is required to produce 0.69 grams of carbon dioxide? The unbalanced equation is shown below: CO (g) + O2 (g) —–> CO2 (g)