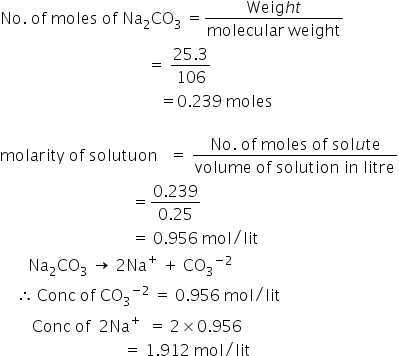25.3 g` sodium carbonate, `Na_(2)CO_(3)`, was dissolved in enough water to make `250 mL` of - YouTube

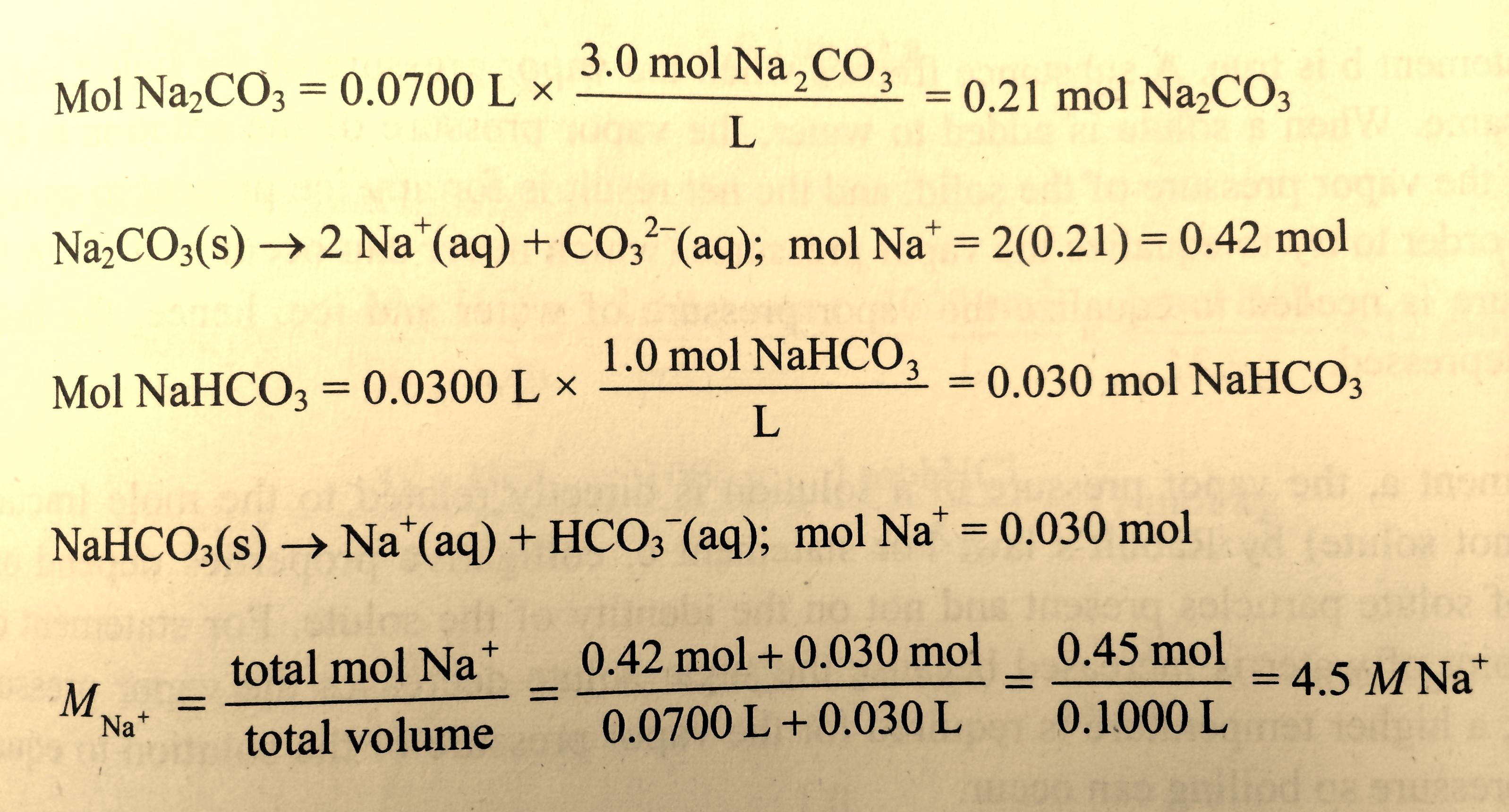
SOLVED: 1.104 of sodium carbonate is dissolved in water in a 250.0 mL volumetric flask; 20.00 mL aliquots of this solution were titrated with nitric acid: An average titre of 23.47 mL

SOLVED: a) What is the concentration of a sodium carbonate solution prepared by dissolving 10.0g of Na2CO3 in water to make 100mL of solution? b) What is the concentration of a sodium

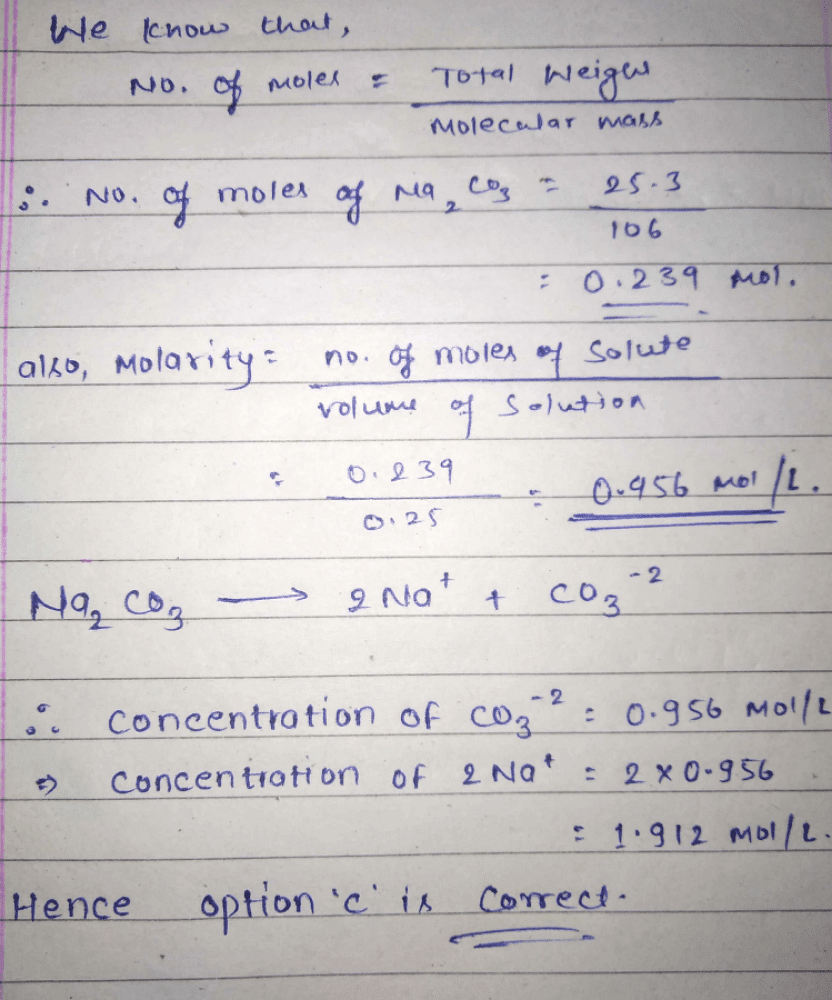
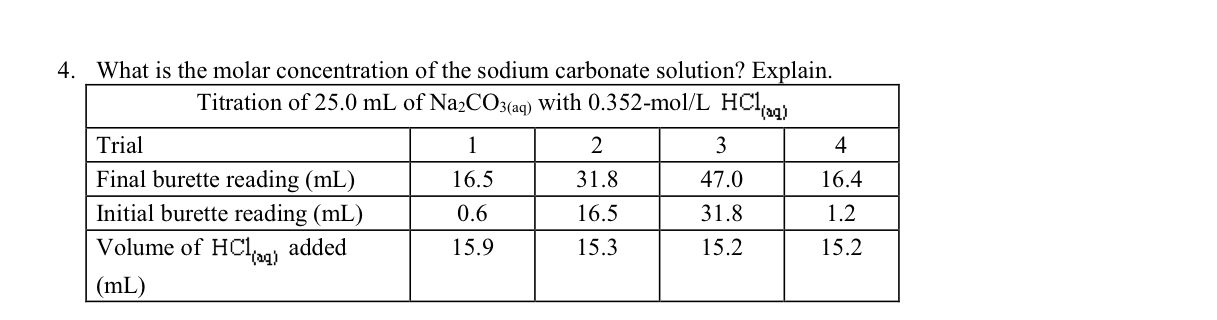
25.3 g of sodium carbonate, Na 2 CO 3 is dissolved in enough water to make 250 mL of solution. If sodium carbonate dissociates completely, the molar concentration of sodium ion, Na ^+

25.3 g of sodium carbonate, Na 2 CO 3 is dissolved in enough water to make 250 mL of solution. If sodium carbonate dissociates completely, the molar concentration of sodium ion, Na ^+

Reaction of Hydrogen Chloride Gas with Sodium Carbonate and Its Deep Removal in a Fixed-Bed Reactor | Industrial & Engineering Chemistry Research
25.3 g of sodium carbonate, Na2CO3 is dissolved in enough water to make 250 mL of solution. - Sarthaks eConnect | Largest Online Education Community

We can measure the concentration of HCl solution by its reaction with pure sodium carbonate. 2 H+ + Na2CO3 ? 2 Na+ + H2O + CO2 Complete reaction with 0.9639 0.0005 g of Na2CO3 required 28.20 0. | Homework.Study.com

a 0.40mol/L solution of sodium carbonate completely dissociates in water, what will be the concentration of - Brainly.com

25.3 g of sodium carbonate Na2CO3 is dissolved in enough water to make 250 mL of solution. If sodium carbonate dissociates completely, molar concentration of sodium ion Na^ + and carbonate ions,

Influence of solution temperature, sodium carbonate concentration, and... | Download Scientific Diagram