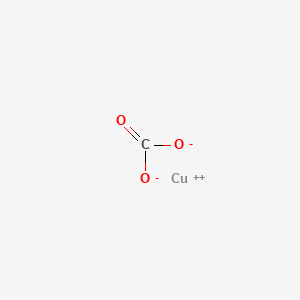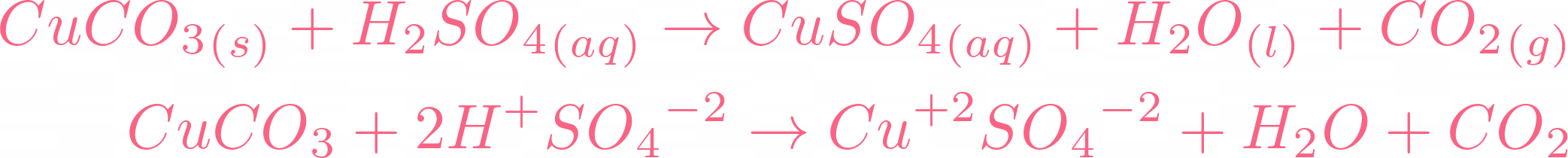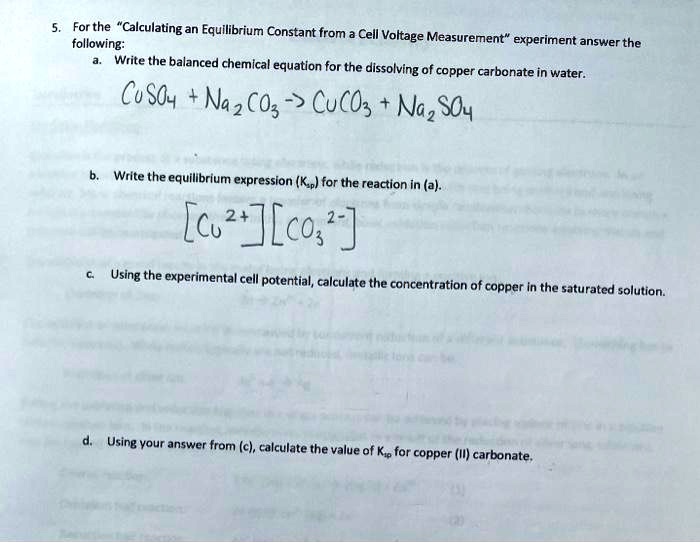
SOLVED: For the "Calculating an Equilibrium Constant from following: Cell Voltage Measurement" experiment answer the Write the balanced chemical equation for the dissolving of = copper carbonate in water: CoSOy NazC C0;

Write a balanced chemical equation for the preparation of the following salt: Copper carbonate |... - YouTube

What is the Difference Between Copper Carbonate and Basic Copper Carbonate | Compare the Difference Between Similar Terms

Write the chemical formula with all the steps for the following: a) Silver nitrate b) Sodium bicarbonate c) Magnesium hydroxide - Science - Atoms and Molecules - 16543201 | Meritnation.com

Given balanced chemical equations to prepare the following salts:Copper chloride using copper carbonate.