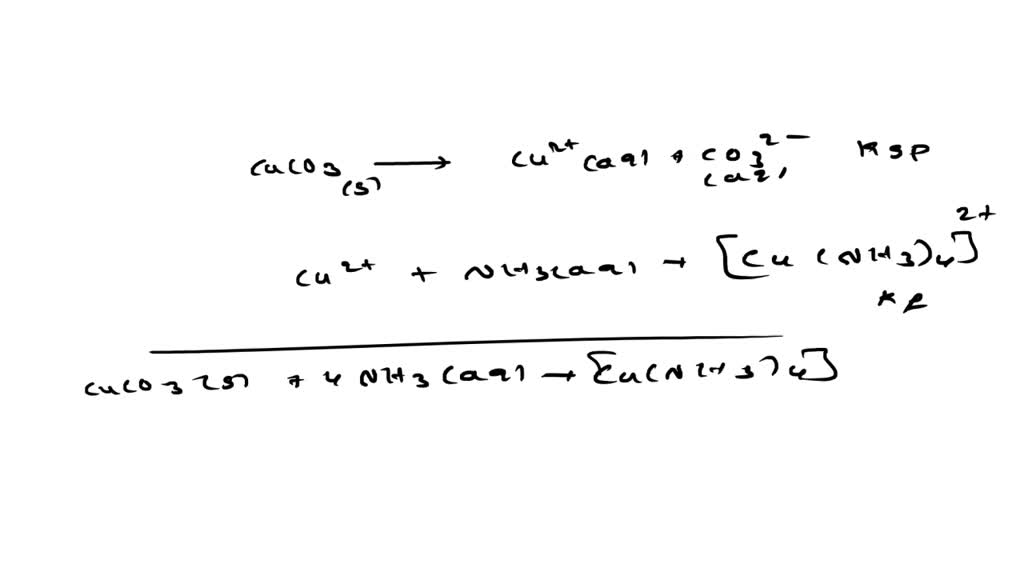Practical: Prepare Copper(II)Sulfate (2.7.8) | Edexcel IGCSE Chemistry Revision Notes 2019 | Save My Exams
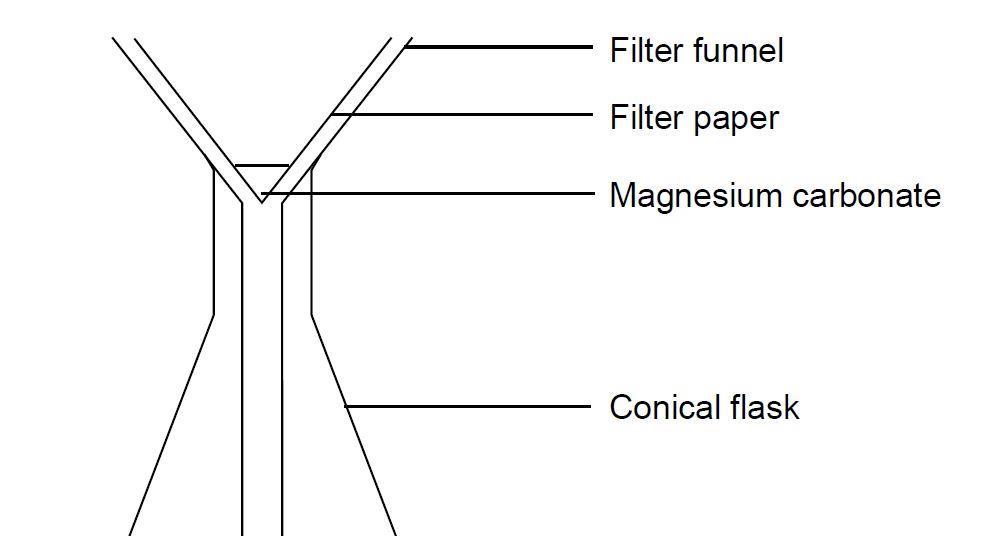
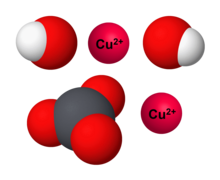
Making magnesium carbonate: the formation of an insoluble salt in water | Experiment | RSC Education
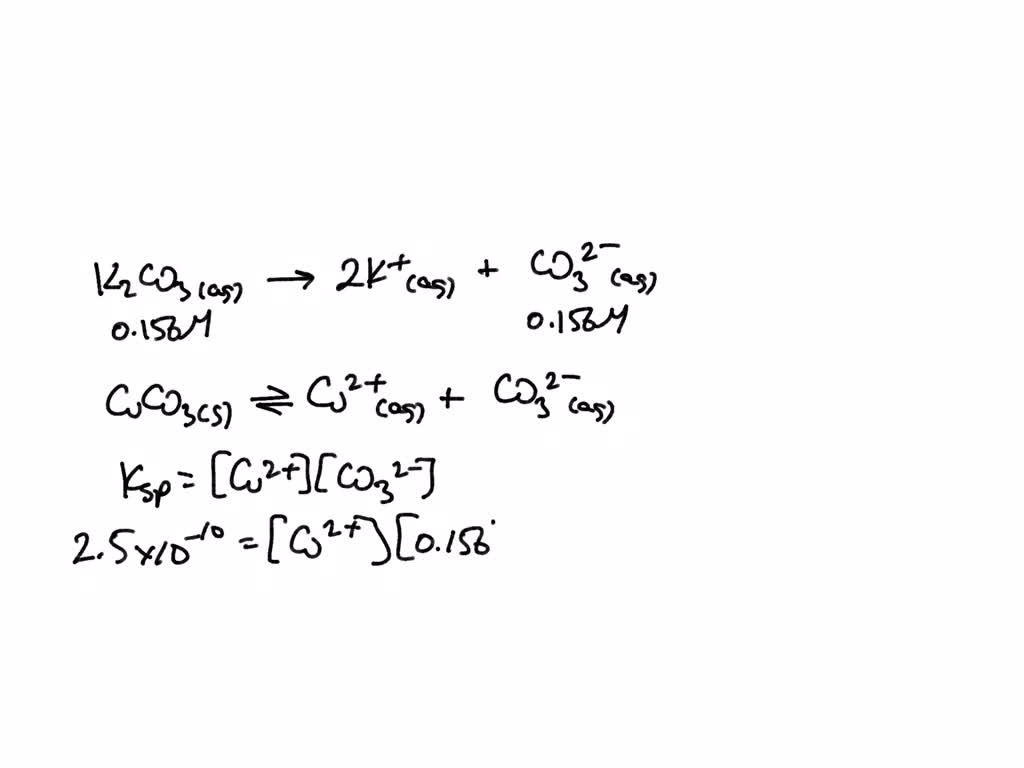
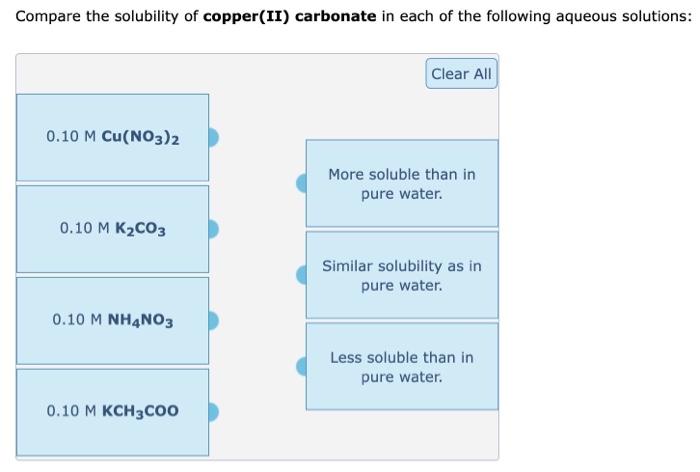
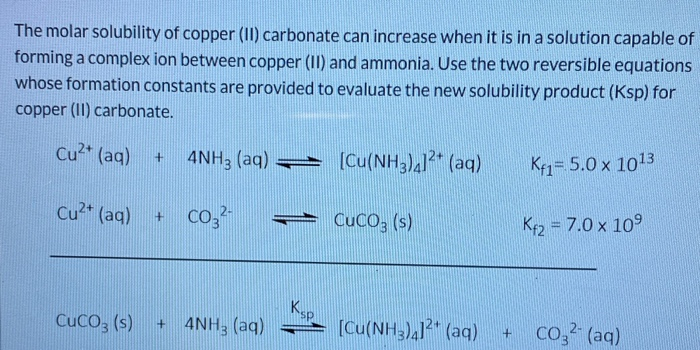
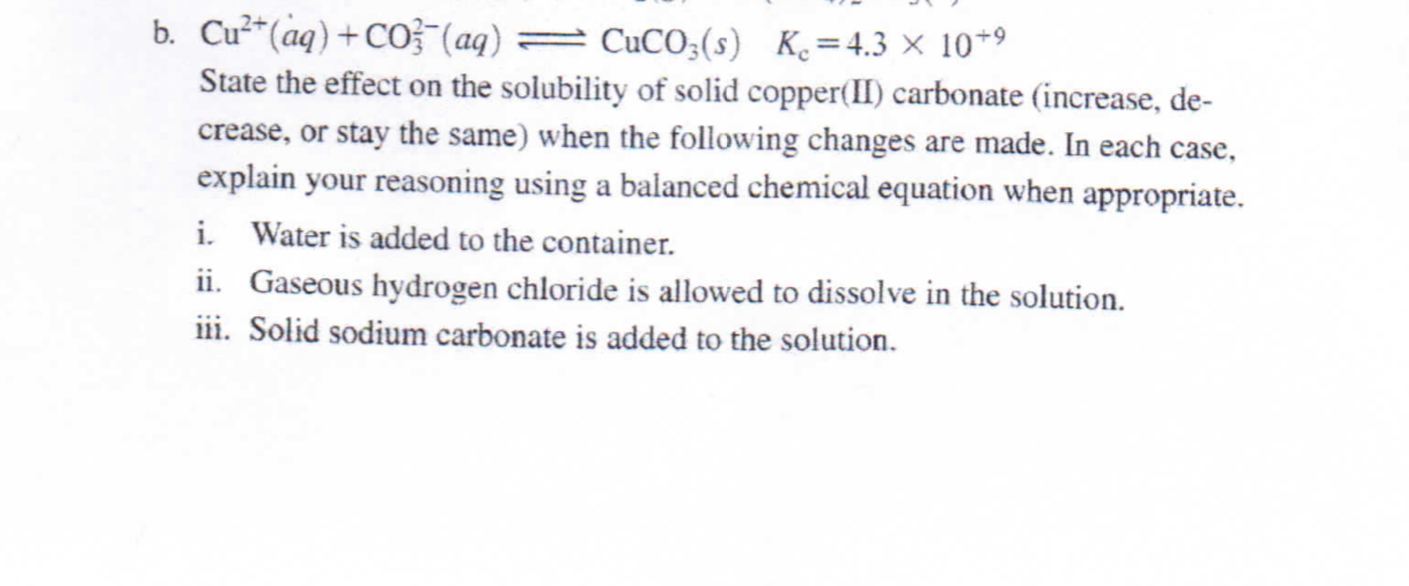
SOLVED: The molar solubility of copper(II) carbonate in a 0.156 M potassium carbonate solution is M. CuCO3 Ksp:2.5 × 10-10

Evaluation of the solubility of a range of copper sources and the effects of iron & sulphur on copper solubility under rumen simulated conditions - ScienceDirect

Molecules | Free Full-Text | Formation of Copper Oxide Nanotextures on Porous Calcium Carbonate Templates for Water Treatment

Copper(II) speciation assuming formation of solid Cu(OH) 2 ; a) DIC=4.8... | Download Scientific Diagram

Copper Carbonate,Senior Chemistry - Extended Experimental In-Industry News-Nickel Acetate,Cobalt Sulfate-Fairsky Industrial Co., Limited

Solutions. Aim:To investigate solubility of some compounds. Method: Water + copper(II) carbonate Water + copper(II) chloride Water + potassium chloride. - ppt download

Solutions. Aim:To investigate solubility of some compounds. Method: Water + copper(II) carbonate Water + copper(II) chloride Water + potassium chloride. - ppt download