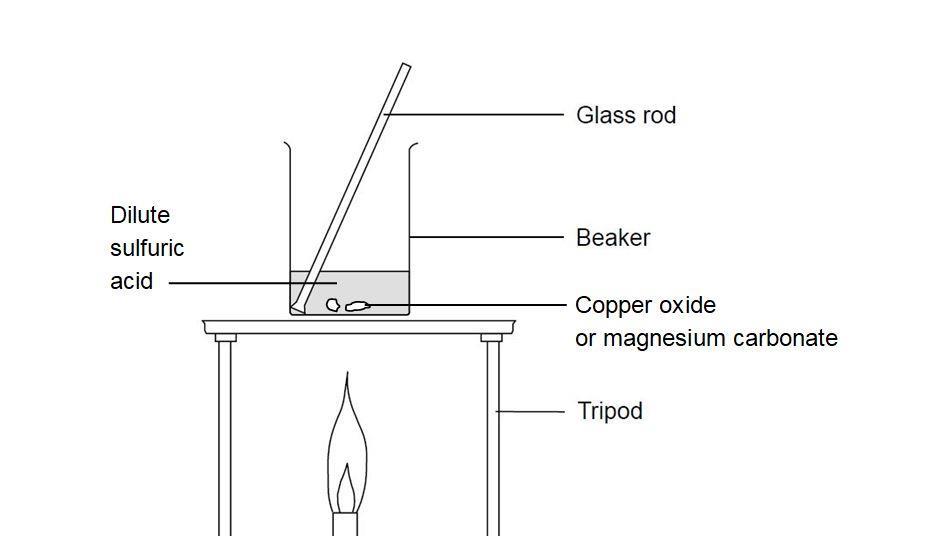
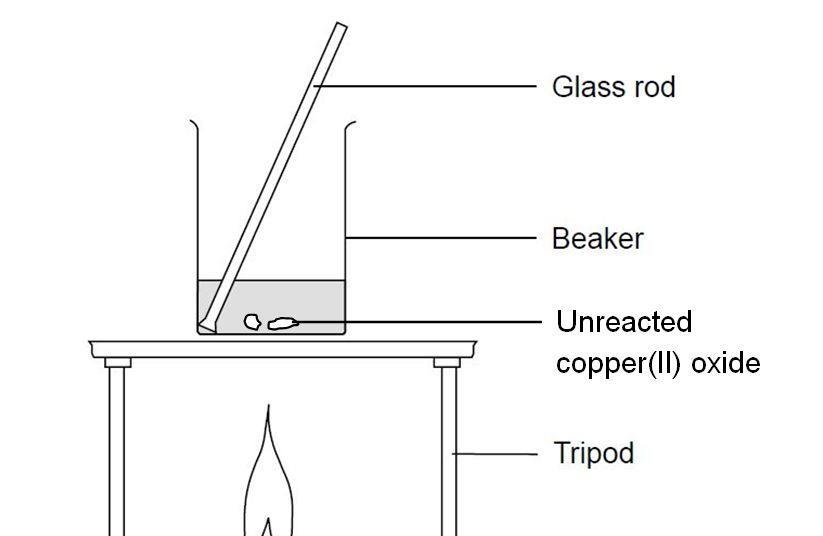
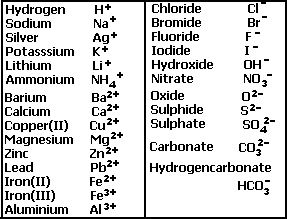
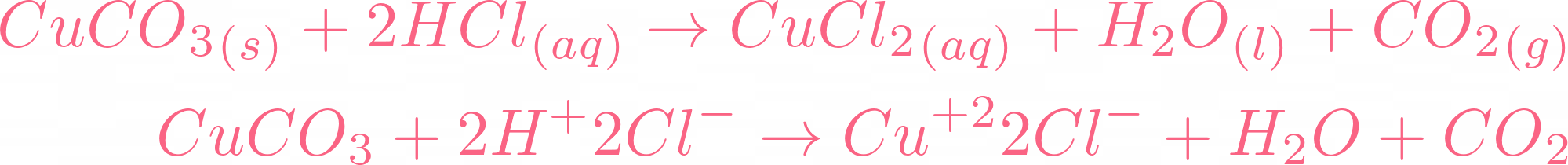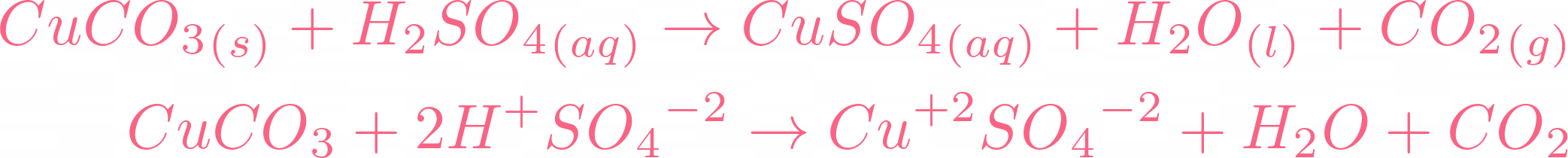![Chemical Reactions to make copper(II) sulfate| Chemistry Basics [Online Video] – O Level Secondary Chemistry Tuition Chemical Reactions to make copper(II) sulfate| Chemistry Basics [Online Video] – O Level Secondary Chemistry Tuition](https://icandochemistry942105908.files.wordpress.com/2021/05/mq2-3.jpg?w=640)
Chemical Reactions to make copper(II) sulfate| Chemistry Basics [Online Video] – O Level Secondary Chemistry Tuition

Practical: Prepare Copper(II)Sulfate (2.7.8) | Edexcel IGCSE Chemistry Revision Notes 2019 | Save My Exams

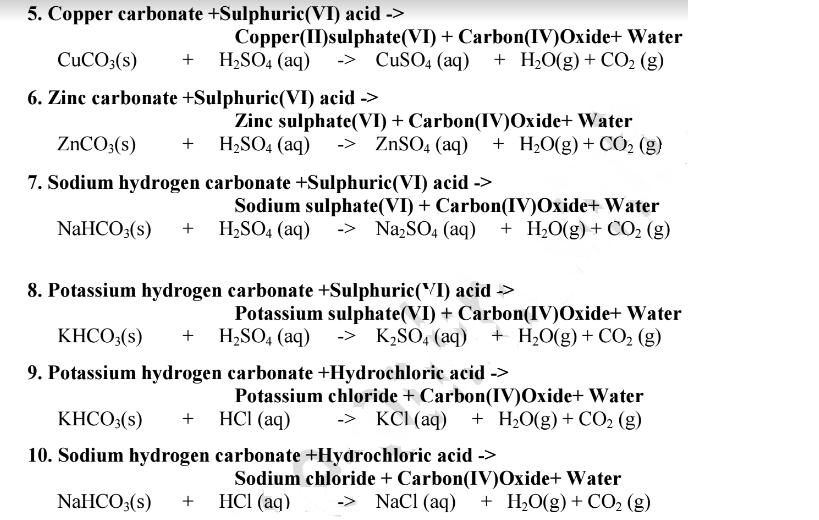
Choosing the substances from the list given below, write balanced chemical equations for the reaction which would be used in the laboratory to obtain the following salts. [Dilute sulphuric acid, Copper, Copper(II)

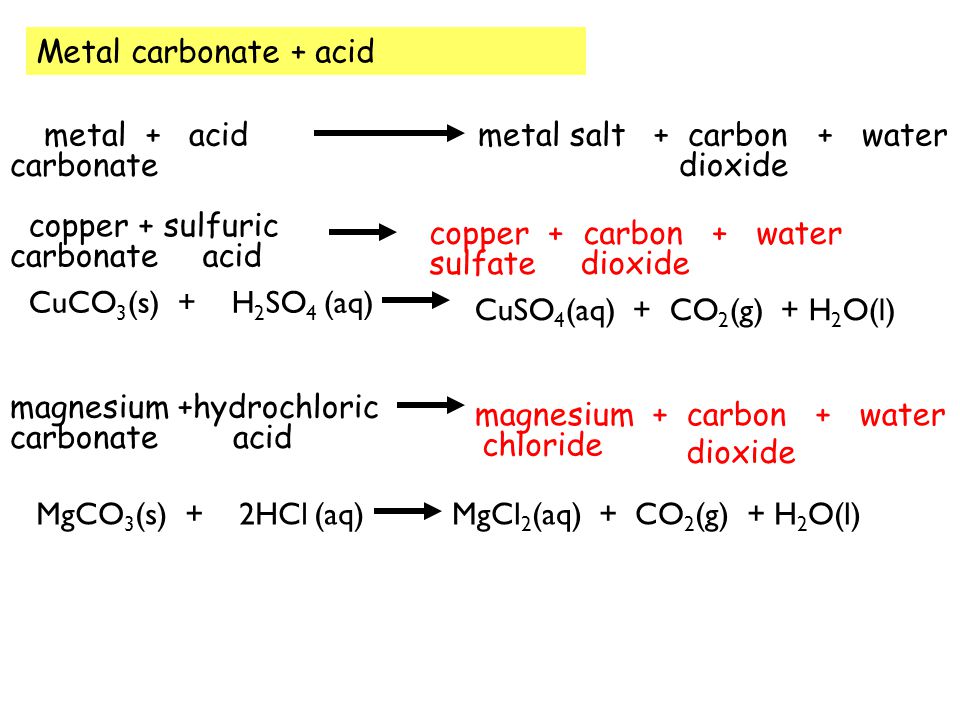
Most carbonates are insoluble (can not be dissolved in water) except those containing sodium or potassium ions. - ppt download
![Chemical Reactions to make copper(II) sulfate| Chemistry Basics [Online Video] – O Level Secondary Chemistry Tuition Chemical Reactions to make copper(II) sulfate| Chemistry Basics [Online Video] – O Level Secondary Chemistry Tuition](http://img.youtube.com/vi/9tJGRn8Su8s/0.jpg)
Chemical Reactions to make copper(II) sulfate| Chemistry Basics [Online Video] – O Level Secondary Chemistry Tuition




![AUFBAU1 [METALS: COPPER COMPOUNDS (2)] AUFBAU1 [METALS: COPPER COMPOUNDS (2)]](https://www.wissensdrang.com/media/scheme2.gif)