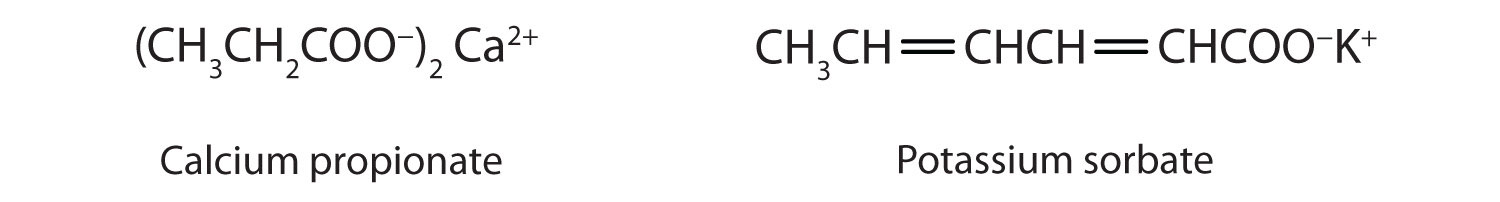
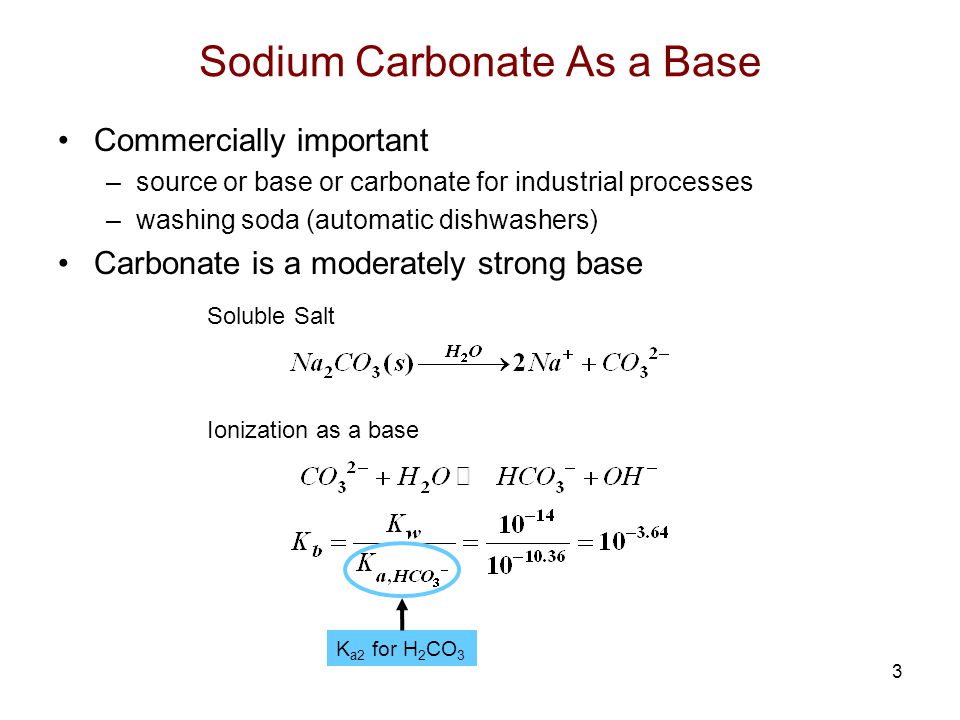
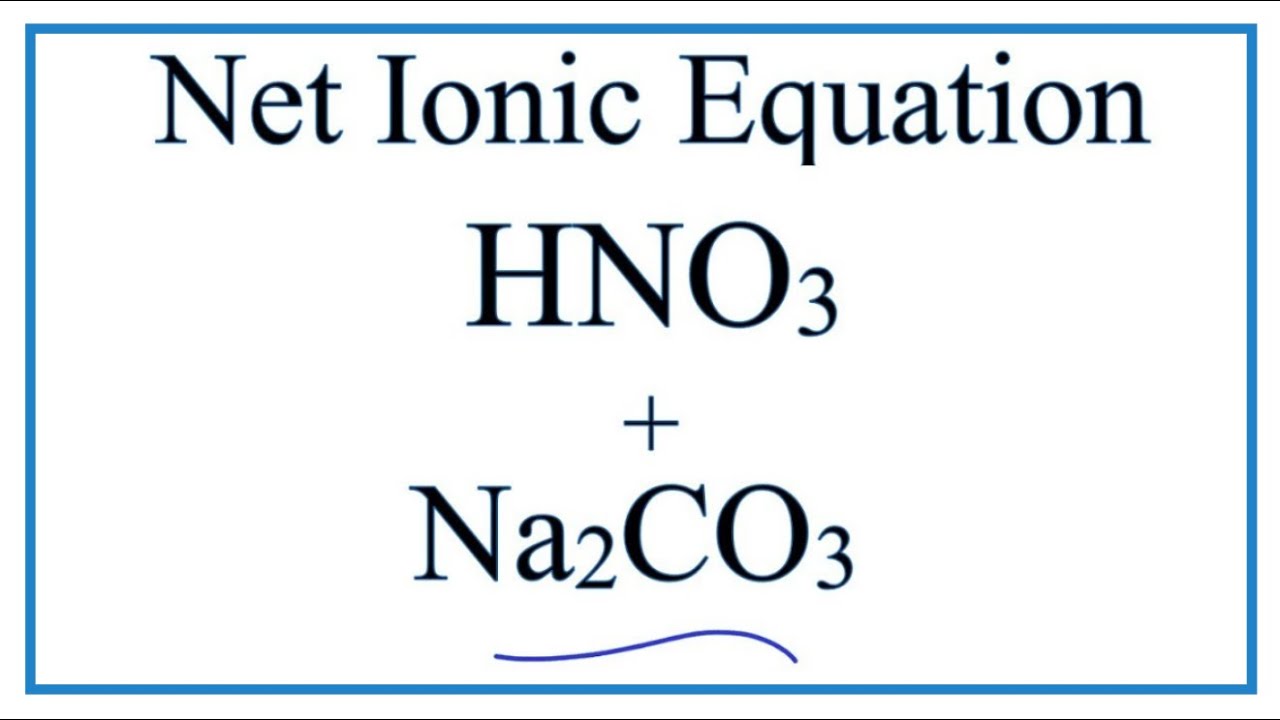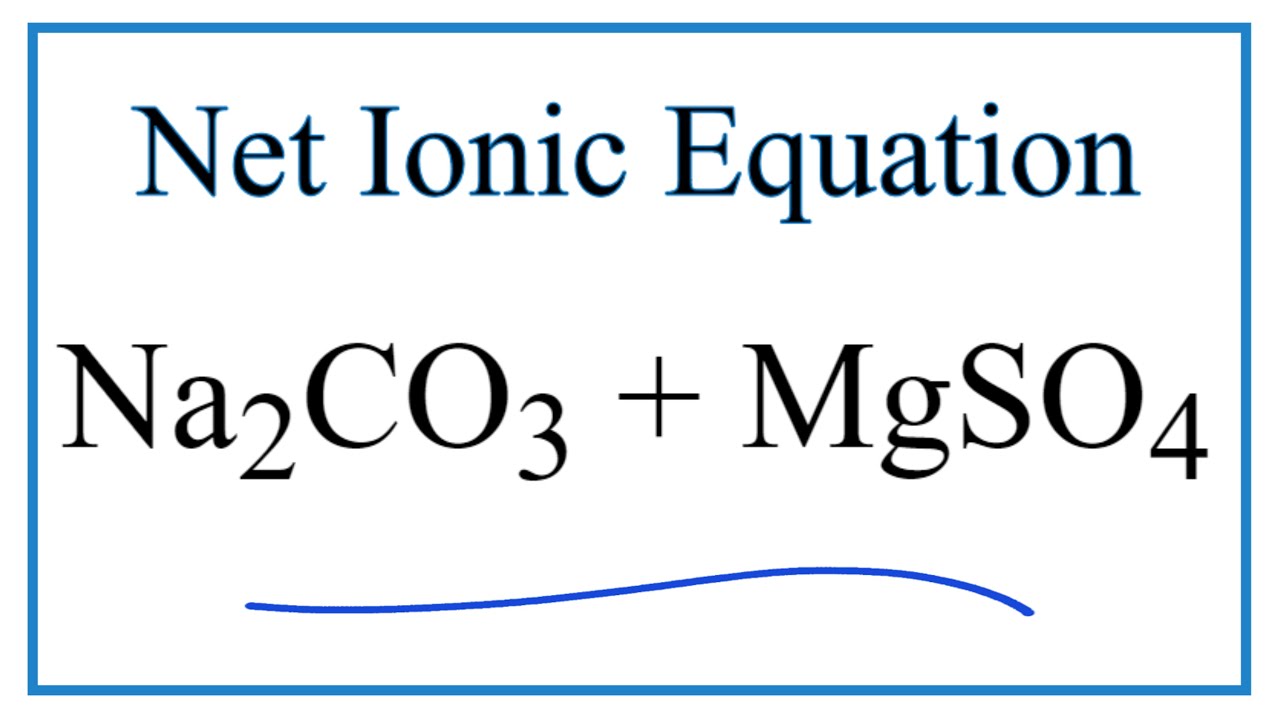97. The conductivity of sodium carbonate solutions at 25° - Journal of the Chemical Society (Resumed) (RSC Publishing)

physical chemistry - Which formula can be used to calculate the exact hydronium concentration present in sodium hydrogen carbonate solution? - Chemistry Stack Exchange
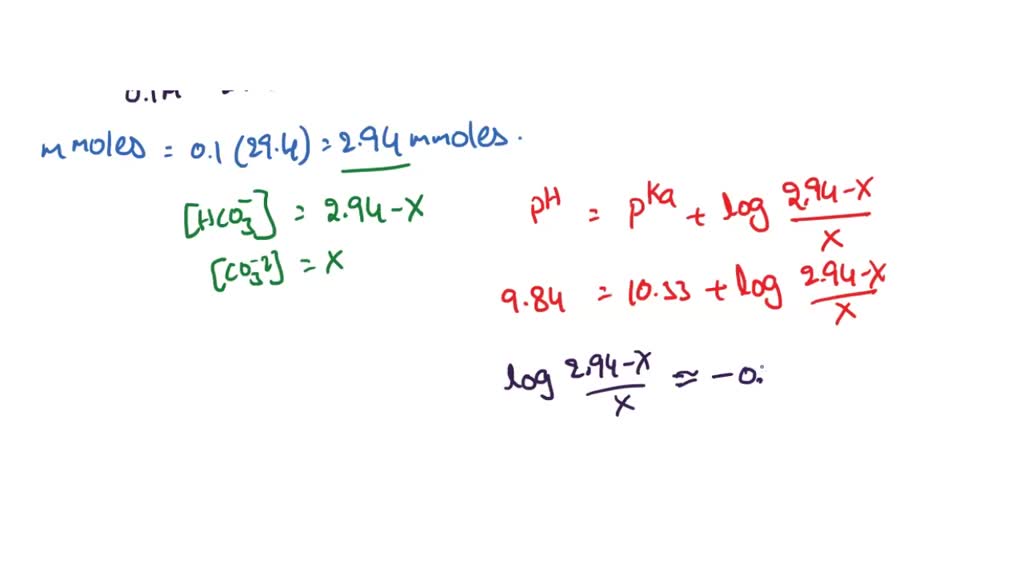
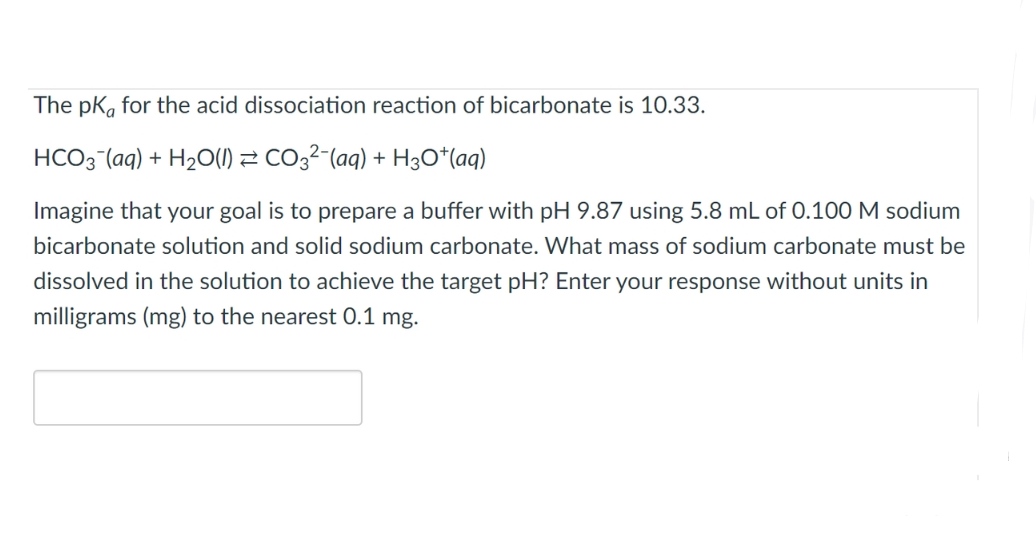
SOLVED: The pKa for the acid dissociation reaction of bicarbonate is 10.33. HCO3–(aq) + H2O(l) ⇄ CO32–(aq) + H3O+(aq) Imagine that your goal is to prepare a buffer with pH 9.84 using